To evaluate blood banks in the Brazilian Amazon region with regard to structure and procedures directed toward the prevention of transfusion-transmitted malaria (TTM).
MethodsThis was a normative evaluation based on the Brazilian National Health Surveillance Agency (ANVISA) Resolution RDC No. 153/2004. Ten blood banks were included in the study and classified as ‘adequate’ (≥80 points), ‘partially adequate’ (from 50 to 80 points), or ‘inadequate’ (<50 points). The following components were evaluated: ‘donor education’ (5 points), ‘clinical screening’ (40 points), ‘laboratory screening’ (40 points) and ‘hemovigilance’ (15 points).
ResultsThe overall median score was 49.8 (minimum=16; maximum=78). Five blood banks were classified as ‘inadequate’ and five as ‘partially adequate’. The median clinical screening score was 26 (minimum=16; maximum=32). The median laboratory screening score was 20 (minimum=0; maximum=32). Eight blood banks performed laboratory tests for malaria; six tested all donations. Seven used thick smears, but only one performed this procedure in accordance with Ministry of Health requirements. One service had a Program of External Quality Evaluation for malaria testing. With regard to hemovigilance, two institutions reported having procedures to detect cases of transfusion-transmitted malaria.
ConclusionMalaria is neglected as a blood–borne disease in the blood banks of the Brazilian Amazon region. None of the institutions were classified as ‘adequate’ in the overall classification or with regard to clinical screening and laboratory screening. Blood bank professionals, the Ministry of Health and Health Surveillance service managers need to pay more attention to this matter so that the safety procedures required by law are complied with.
Malaria is endemic in the Brazilian Amazonian region and is mainly transmitted by vectors. Transfusion-transmitted malaria (TTM) is, however, one of the most important blood–borne parasitic diseases and represents a significant risk for patients in endemic areas as cases are usually very severe.1,2 In the United States, malaria is not endemic with only three TTM cases being reported on average per annum.2 In Brazil, the National Hemovigilance System, created in 2002,3 has thus far registered four TTM cases, three in 2006 and one in 2007. By using nucleic acid tests (NAT) to detect Plasmodium in endemic areas in Brazil, the prevalence of malaria in eligible donors is estimated to be between 0.3% and 3%.4–6 It is therefore likely that TTM is underreported to the National Hemovigilance System.
In Brazil, the Health Surveillance service (VISA) works to control transfusion risk and blood quality through actions such as standard regulations, inspection, health education and hemovigilance. These measures are carried out by VISA at federal, state and municipal levels.7 The technical and sanitary requirements for blood banks (BBs) to prevent TTM have been defined since 1988 by Law 7649/19888 and other regulations – Decrees, Ministry of Health (MoH) Ordinances and Resolutions of the Collegiate Directorate (RDC) of the National Health Surveillance Agency (ANVISA).
In 1989, the MoH Ordinance No. 721/GM/19899 introduced the requirement for donor clinical and epidemiological screening, including history of previous infections, recent signs and symptoms and travel to endemic areas. During regulatory reviews, two substantial updates were included by ANVISA RDC No. 343/2002,10 namely: BBs in endemic areas must use the annual parasite index (API) to exclude donors who visit high-risk areas (API >49.9 cases/1000 inhabitants) and must perform laboratory tests on eligible donors. All subsequent regulations have maintained these obligations with small modifications.
This study presents a normative evaluation of selected BBs in the Brazilian Amazon region with the aim of preventing TTM. The study's objective was to analyze the adherence of these BBs to prevailing regulations and standards, to describe and discuss the current practices of these establishments and to recommend measures to improve the prevention of TTM.
MethodsThis was a normative evaluation study that focused on an evaluation to inform management.11 According to Contandriopoulos et al.,12 to evaluate is to make a value judgment about an intervention or its components in order to assist decision making. The judgment can be based on the application of criteria and regulations and, in this case, it is called a normative evaluation. This, in turn, is defined as the act of making a judgment, comparing the organization (structure), the procedure or methods developed (process), the resources deployed and the results obtained with the requirements and criteria established by regulations. It is a scientific activity that must be carried out with methodological rigor.13
This study used external evaluators with the evaluation being performed in a natural context (without interventions).12 The evaluation was based on ANVISA RDC No. 153/200414 (RDC 153/2004), in force at the time, regarding the issue of TTM prevention. The components evaluated were structure and process.15
Data collection took place between January 2009 and June 2011. All nine Coordinator BBs responsible for the Brazilian Amazon region and one hemotherapy nucleus were evaluated. A standardized semi-structured list of questions was used to collect data by interviewing the person technically responsible for the BB and/or the person responsible for the sectors/activities evaluated. Donor education, clinical screening, laboratory screening and hemovigilance were evaluated. The interviews were conducted by just one researcher with experience in administering inspection questionnaires and epidemiology interviewing. In addition to the interview, information was collected from educational materials for donors and standards operational procedures (SOP).
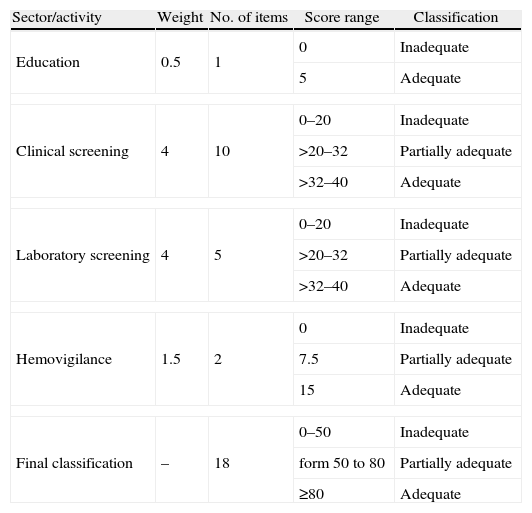
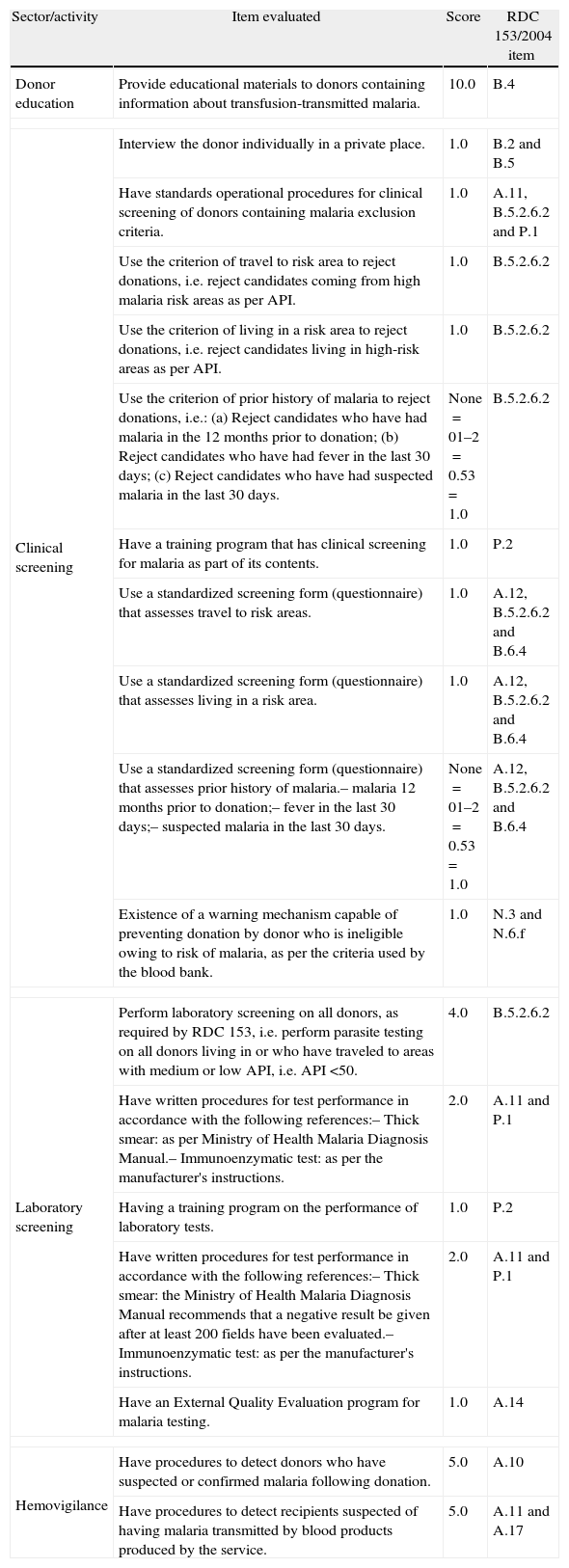
The BBs were classified as adequate, partially adequate or inadequate (Table 1), depending on the score obtained. The concept of potential risk16 was defined as ‘the possibility of the occurrence of a disease, whilst not necessarily describing the disease or the likelihood of its occurrence. This concept which expresses the value judgment about potential exposure to a possible risk was used to define the weighting of each activity and item. Different to epidemiological risk which can be measured and calculated, potential risk is often determined based on the cumulative perception of specialists regarding the defects or faults in a given product, process or service over time.16 The criteria for the scores were therefore based on the authors’ assessment of the potential risk of TTM cases occurring owing to non-compliance with the items of the norms. Clinical and laboratory screening were considered to be critical for the regulation of TTM. The details of the scores for each item are shown in Table 2. Although the criteria are based on RDC 153/2004, the final text of the criteria for each item evaluated expresses the specific focus on controlling the risk of TTM. The criteria are not therefore literal transcriptions of the requirements in RDC 153/2004.
Description of the model for classifying sectors/activities and blood banks including scores obtained following evaluation.
| Sector/activity | Weight | No. of items | Score range | Classification |
| Education | 0.5 | 1 | 0 | Inadequate |
| 5 | Adequate | |||
| Clinical screening | 4 | 10 | 0–20 | Inadequate |
| >20–32 | Partially adequate | |||
| >32–40 | Adequate | |||
| Laboratory screening | 4 | 5 | 0–20 | Inadequate |
| >20–32 | Partially adequate | |||
| >32–40 | Adequate | |||
| Hemovigilance | 1.5 | 2 | 0 | Inadequate |
| 7.5 | Partially adequate | |||
| 15 | Adequate | |||
| Final classification | – | 18 | 0–50 | Inadequate |
| form 50 to 80 | Partially adequate | |||
| ≥80 | Adequate | |||
Details of the criteria assessed for each blood bank sector and activity, their scores and the RDC 153/2004 item used as a basis for the requirement.
| Sector/activity | Item evaluated | Score | RDC 153/2004 item |
| Donor education | Provide educational materials to donors containing information about transfusion-transmitted malaria. | 10.0 | B.4 |
| Clinical screening | Interview the donor individually in a private place. | 1.0 | B.2 and B.5 |
| Have standards operational procedures for clinical screening of donors containing malaria exclusion criteria. | 1.0 | A.11, B.5.2.6.2 and P.1 | |
| Use the criterion of travel to risk area to reject donations, i.e. reject candidates coming from high malaria risk areas as per API. | 1.0 | B.5.2.6.2 | |
| Use the criterion of living in a risk area to reject donations, i.e. reject candidates living in high-risk areas as per API. | 1.0 | B.5.2.6.2 | |
| Use the criterion of prior history of malaria to reject donations, i.e.: (a) Reject candidates who have had malaria in the 12 months prior to donation; (b) Reject candidates who have had fever in the last 30 days; (c) Reject candidates who have had suspected malaria in the last 30 days. | None=01–2=0.53=1.0 | B.5.2.6.2 | |
| Have a training program that has clinical screening for malaria as part of its contents. | 1.0 | P.2 | |
| Use a standardized screening form (questionnaire) that assesses travel to risk areas. | 1.0 | A.12, B.5.2.6.2 and B.6.4 | |
| Use a standardized screening form (questionnaire) that assesses living in a risk area. | 1.0 | A.12, B.5.2.6.2 and B.6.4 | |
| Use a standardized screening form (questionnaire) that assesses prior history of malaria.– malaria 12 months prior to donation;– fever in the last 30 days;– suspected malaria in the last 30 days. | None=01–2=0.53=1.0 | A.12, B.5.2.6.2 and B.6.4 | |
| Existence of a warning mechanism capable of preventing donation by donor who is ineligible owing to risk of malaria, as per the criteria used by the blood bank. | 1.0 | N.3 and N.6.f | |
| Laboratory screening | Perform laboratory screening on all donors, as required by RDC 153, i.e. perform parasite testing on all donors living in or who have traveled to areas with medium or low API, i.e. API <50. | 4.0 | B.5.2.6.2 |
| Have written procedures for test performance in accordance with the following references:– Thick smear: as per Ministry of Health Malaria Diagnosis Manual.– Immunoenzymatic test: as per the manufacturer's instructions. | 2.0 | A.11 and P.1 | |
| Having a training program on the performance of laboratory tests. | 1.0 | P.2 | |
| Have written procedures for test performance in accordance with the following references:– Thick smear: the Ministry of Health Malaria Diagnosis Manual recommends that a negative result be given after at least 200 fields have been evaluated.– Immunoenzymatic test: as per the manufacturer's instructions. | 2.0 | A.11 and P.1 | |
| Have an External Quality Evaluation program for malaria testing. | 1.0 | A.14 | |
| Hemovigilance | Have procedures to detect donors who have suspected or confirmed malaria following donation. | 5.0 | A.10 |
| Have procedures to detect recipients suspected of having malaria transmitted by blood products produced by the service. | 5.0 | A.11 and A.17 | |
API: annual parasite index.
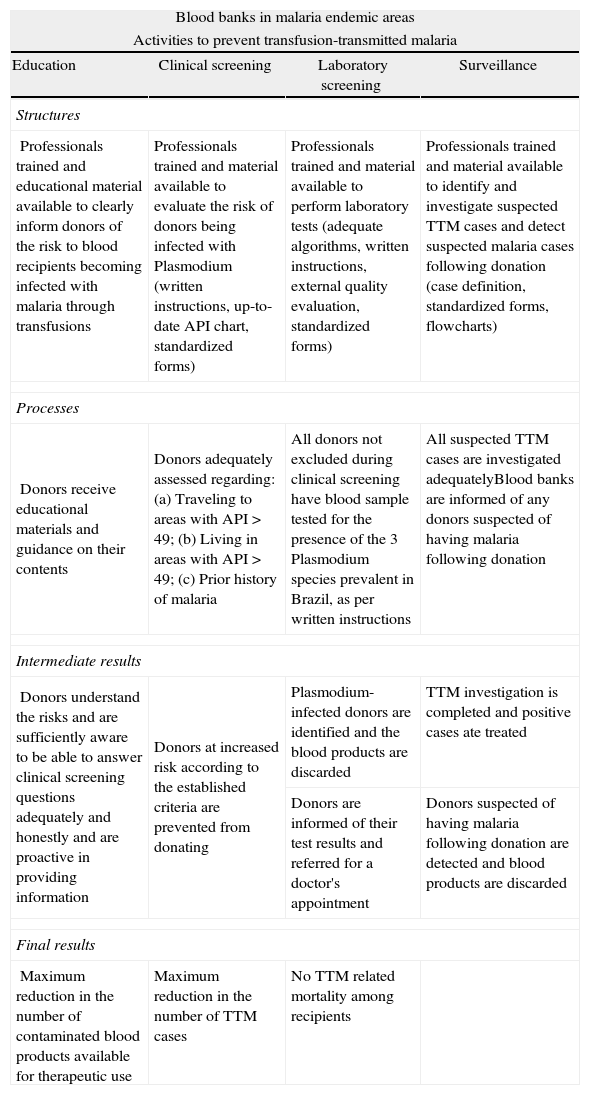
The ideal model of best practices for ensuring maximum TTM risk reduction, according to RDC 153/2004, is shown in Table 3. In this model the four activities involved in TTM prevention are shown as they relate to structures, processes and results.
The ideal model of best practices to ensure maximum reduction of transfusion-transmitted malaria risk according to RDC 153/2004.
| Blood banks in malaria endemic areas | |||
| Activities to prevent transfusion-transmitted malaria | |||
| Education | Clinical screening | Laboratory screening | Surveillance |
| Structures | |||
| Professionals trained and educational material available to clearly inform donors of the risk to blood recipients becoming infected with malaria through transfusions | Professionals trained and material available to evaluate the risk of donors being infected with Plasmodium (written instructions, up-to-date API chart, standardized forms) | Professionals trained and material available to perform laboratory tests (adequate algorithms, written instructions, external quality evaluation, standardized forms) | Professionals trained and material available to identify and investigate suspected TTM cases and detect suspected malaria cases following donation (case definition, standardized forms, flowcharts) |
| Processes | |||
| Donors receive educational materials and guidance on their contents | Donors adequately assessed regarding: (a) Traveling to areas with API > 49; (b) Living in areas with API > 49; (c) Prior history of malaria | All donors not excluded during clinical screening have blood sample tested for the presence of the 3 Plasmodium species prevalent in Brazil, as per written instructions | All suspected TTM cases are investigated adequatelyBlood banks are informed of any donors suspected of having malaria following donation |
| Intermediate results | |||
| Donors understand the risks and are sufficiently aware to be able to answer clinical screening questions adequately and honestly and are proactive in providing information | Donors at increased risk according to the established criteria are prevented from donating | Plasmodium-infected donors are identified and the blood products are discarded | TTM investigation is completed and positive cases ate treated |
| Donors are informed of their test results and referred for a doctor's appointment | Donors suspected of having malaria following donation are detected and blood products are discarded | ||
| Final results | |||
| Maximum reduction in the number of contaminated blood products available for therapeutic use | Maximum reduction in the number of TTM cases | No TTM related mortality among recipients | |
TTM: transfusion-transmitted malaria; API: annual parasite index.
This study was approved by the Research Ethics Committees of the Júlio Müller University Hospital of the Universidade Federal de Mato Grosso and the Medicine School of the Universidade de Brasília.
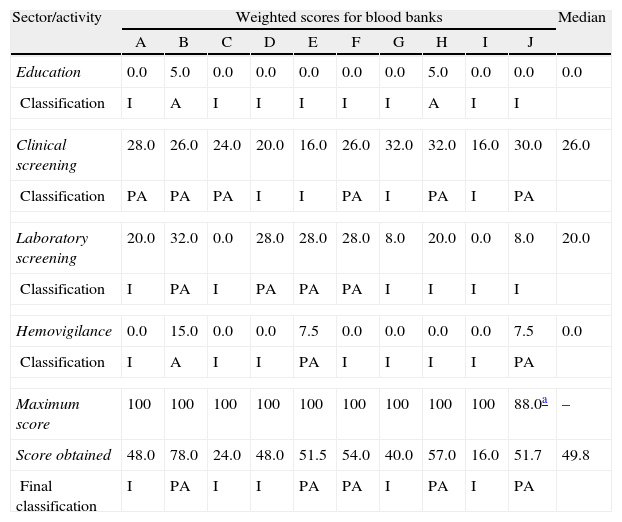
ResultsThe final median score for the BBs as a whole was 49.8 (minimum=16; maximum=78) (Table 4). None of the BBs were considered ‘Adequate’; five were classified as ‘Partially Adequate’ and five as ‘Inadequate’. Table 5 provides detailed results of the components and items.
Final score obtained by each sector/activity and classification of the ten blood banks according to the criteria established in this evaluation.
| Sector/activity | Weighted scores for blood banks | Median | |||||||||
| A | B | C | D | E | F | G | H | I | J | ||
| Education | 0.0 | 5.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.0 | 0.0 | 0.0 | 0.0 |
| Classification | I | A | I | I | I | I | I | A | I | I | |
| Clinical screening | 28.0 | 26.0 | 24.0 | 20.0 | 16.0 | 26.0 | 32.0 | 32.0 | 16.0 | 30.0 | 26.0 |
| Classification | PA | PA | PA | I | I | PA | I | PA | I | PA | |
| Laboratory screening | 20.0 | 32.0 | 0.0 | 28.0 | 28.0 | 28.0 | 8.0 | 20.0 | 0.0 | 8.0 | 20.0 |
| Classification | I | PA | I | PA | PA | PA | I | I | I | I | |
| Hemovigilance | 0.0 | 15.0 | 0.0 | 0.0 | 7.5 | 0.0 | 0.0 | 0.0 | 0.0 | 7.5 | 0.0 |
| Classification | I | A | I | I | PA | I | I | I | I | PA | |
| Maximum score | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 88.0a | – |
| Score obtained | 48.0 | 78.0 | 24.0 | 48.0 | 51.5 | 54.0 | 40.0 | 57.0 | 16.0 | 51.7 | 49.8 |
| Final classification | I | PA | I | I | PA | PA | I | PA | I | PA | |
I: inadequate; PA: partially adequate; A: adequate.
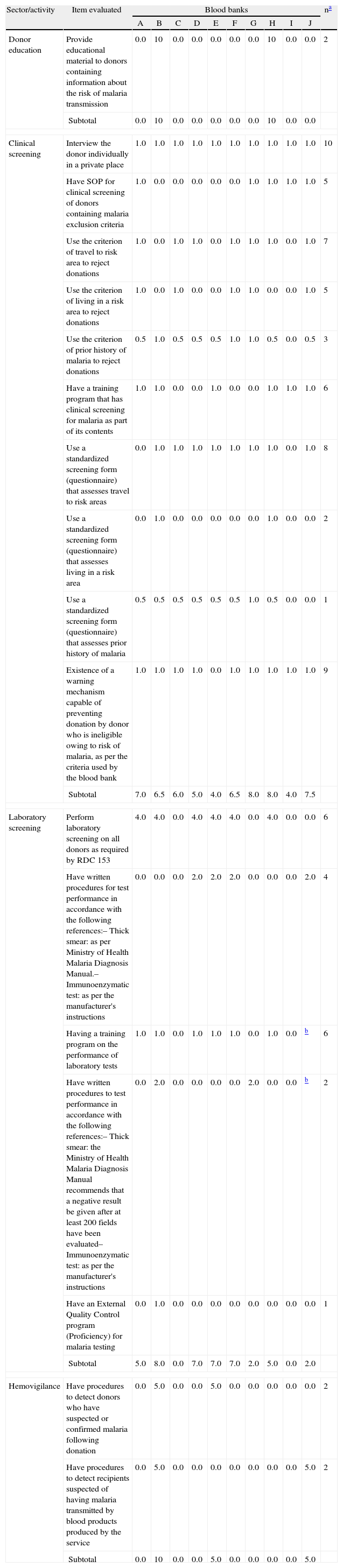
Scores obtained for each item evaluated at the ten blood banks studied (A–J) in the Brazilian Amazon region.
| Sector/activity | Item evaluated | Blood banks | na | |||||||||
| A | B | C | D | E | F | G | H | I | J | |||
| Donor education | Provide educational material to donors containing information about the risk of malaria transmission | 0.0 | 10 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 10 | 0.0 | 0.0 | 2 |
| Subtotal | 0.0 | 10 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 10 | 0.0 | 0.0 | ||
| Clinical screening | Interview the donor individually in a private place | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 10 |
| Have SOP for clinical screening of donors containing malaria exclusion criteria | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 1.0 | 1.0 | 5 | |
| Use the criterion of travel to risk area to reject donations | 1.0 | 0.0 | 1.0 | 1.0 | 0.0 | 1.0 | 1.0 | 1.0 | 0.0 | 1.0 | 7 | |
| Use the criterion of living in a risk area to reject donations | 1.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | 1.0 | 0.0 | 0.0 | 1.0 | 5 | |
| Use the criterion of prior history of malaria to reject donations | 0.5 | 1.0 | 0.5 | 0.5 | 0.5 | 1.0 | 1.0 | 0.5 | 0.0 | 0.5 | 3 | |
| Have a training program that has clinical screening for malaria as part of its contents | 1.0 | 1.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | 1.0 | 1.0 | 6 | |
| Use a standardized screening form (questionnaire) that assesses travel to risk areas | 0.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.0 | 1.0 | 8 | |
| Use a standardized screening form (questionnaire) that assesses living in a risk area | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 2 | |
| Use a standardized screening form (questionnaire) that assesses prior history of malaria | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1.0 | 0.5 | 0.0 | 0.0 | 1 | |
| Existence of a warning mechanism capable of preventing donation by donor who is ineligible owing to risk of malaria, as per the criteria used by the blood bank | 1.0 | 1.0 | 1.0 | 1.0 | 0.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 9 | |
| Subtotal | 7.0 | 6.5 | 6.0 | 5.0 | 4.0 | 6.5 | 8.0 | 8.0 | 4.0 | 7.5 | ||
| Laboratory screening | Perform laboratory screening on all donors as required by RDC 153 | 4.0 | 4.0 | 0.0 | 4.0 | 4.0 | 4.0 | 0.0 | 4.0 | 0.0 | 0.0 | 6 |
| Have written procedures for test performance in accordance with the following references:– Thick smear: as per Ministry of Health Malaria Diagnosis Manual.– Immunoenzymatic test: as per the manufacturer's instructions | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 4 | |
| Having a training program on the performance of laboratory tests | 1.0 | 1.0 | 0.0 | 1.0 | 1.0 | 1.0 | 0.0 | 1.0 | 0.0 | b | 6 | |
| Have written procedures to test performance in accordance with the following references:– Thick smear: the Ministry of Health Malaria Diagnosis Manual recommends that a negative result be given after at least 200 fields have been evaluated– Immunoenzymatic test: as per the manufacturer's instructions | 0.0 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 | 0.0 | b | 2 | |
| Have an External Quality Control program (Proficiency) for malaria testing | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1 | |
| Subtotal | 5.0 | 8.0 | 0.0 | 7.0 | 7.0 | 7.0 | 2.0 | 5.0 | 0.0 | 2.0 | ||
| Hemovigilance | Have procedures to detect donors who have suspected or confirmed malaria following donation | 0.0 | 5.0 | 0.0 | 0.0 | 5.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2 |
| Have procedures to detect recipients suspected of having malaria transmitted by blood products produced by the service | 0.0 | 5.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.0 | 2 | |
| Subtotal | 0.0 | 10 | 0.0 | 0.0 | 5.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.0 | ||
With regard to the donor education component, all BBs only used leaflets and posters to inform donors about blood–borne diseases. Malaria was mentioned in leaflets in only two of the ten BBs (Table 4). Four types of leaflets produced by the MoH were found in two BBs, but none of them mentioned TTM.
Six BBs were considered partially adequate and four were considered inadequate in relation to clinical screening (Table 4). Five BBs had SOPs for clinical screening of malaria but only one used a standardized screening form containing all the items required by the regulation to evaluate prior history of malaria (fever, suspected malaria and have had malaria) (Table 5).
Seven BBs used exclusion criteria in cases of exposure to risk areas (Table 5) based on API rates and all of them defined high-risk areas as the municipal. One BB also took district API rates into consideration when the municipal in question was the state capital city. Four different ineligibility periods were identified for donors who had traveled to high-risk areas, namely 7 (1/7), 15 (1/7), 30 (2/7) and 180 (3/7) days. The Epidemiological Surveillance Service provides six of the ten BBs with the API rates and one of the ten BBs directly accessed the API rates in the Information System for Malaria in Endemic Regions (SIVEP-Malaria). All of them used API for the preceding year to define high-risk areas in the current year.
With regard to exclusion owing to prior history of malaria, only one BB did not apply any of the criteria established by the regulation and three applied all the required criteria (Table 5). Nine BBs applied the criterion ‘had malaria in the last 12 months’, seven applied the criterion ‘fever in the last 30 days’ and three applied the criterion ‘suspected malaria in the last 30 days’. Two BBs used these last two criteria considering a period of 15 days rather than 30. Other criteria mentioned were ‘having taken (or taking) medication for malaria’ (2 BBs) and ‘someone at home has got malaria’ (2 BBs).
Analysis of the screening form showed that only one BB used a form containing the three exclusion criteria for prior history of malaria, whilst two BBs had no question for these criteria (Table 5). Considerable variations were observed in the questions on the screening forms regarding having traveled to and living in high-risk areas, namely: (1) ‘Have you been in a high malaria risk area?’; (2) ‘Have you been in a high malaria risk area? Where? How long ago?’; (3) ‘Have you traveled to a different municipality within the state? When and which one?’; (4) ‘Have you traveled anywhere in the last six months? If yes, where?’; (5) ‘Do you come from an endemic malaria zone?’; (6) ‘Have you been in an endemic malaria region in the last 30 days?’; (7) ‘Do you live in a high malaria risk area?’; and (8) ‘Do you live in a high malaria risk area? Where? For how long?’. With the exception of questions 3 and 4, it is possible to interpret that the responsibility for indicating exposure to malaria risk areas was placed on the donor.
In relation to laboratory screening for malaria, four BBs were classified as partially adequate and seven as inadequate (Table 4). Only six BBs performed laboratory screening on all donations in accordance with the regulation requirements (Table 5). Two BBs only tested donors exposed to areas with API between 10.0 and 49.9 cases per 1000 inhabitants (medium risk) and a further two did not perform any test at all, arguing that they were not in the endemic area. Seven BBs used thick smears in laboratory screening and one used immunochromatographic testing. With regard to SOPs for the performance of laboratory tests (thick blood smear or immunochromatographic tests), two BBs were considered to have adequate instructions. One of them performed thick blood smear testing and the instructions adequately stated that 200 fields should be evaluated before establishing a negative result.17
When considering the eight BBs that performed some type of laboratory test for malaria (whether or not it complied adequately with the regulations), six reported not having identified any positive tests in the last five years. Furthermore, only one BB reported having a malaria test External Quality Evaluation Program (EQE) (Table 5). On the other hand, all the participating BBs had EQE for the remaining laboratory screening tests and for immunohematology.
With regard to the hemovigilance component, an innovative experiment was encountered in one BB with the inclusion of the following text in the information leaflet given to each donor and entitled ‘Care after donating blood’: ‘I have received guidance that if I have symptoms of malaria/dengue and/or fever within ___ days of donating blood, I must inform the _____ Blood Bank by calling ___ (telephone number) ___’.
Two BBs reported having detected cases of TTM resulting from blood products produced by them. All these cases died.
DiscussionPublications about the evaluation of Brazilian BBs are rare and, when they are found, their themes are donor satisfaction,18 serological test performance19 or costs.20 This is the first Brazilian study to evaluate the quality of the procedures and methods used by BBs to prevent TTM.
Overall performance registered by the study shows that adherence to RDC 153/2004 with regard to the prevention of TTM is neglected by the participating BBs. Only one establishment obtained more than 60 points on the proposed scoring system.
Donor education is an important factor for reducing serological ineligibility and disease transmission through transfusions.21 Although the participating BBs used leaflets as an initiative aimed at donor education, most of the leaflets made no reference to TTM. This strengthens the argument that malaria is usually neglected as a risk for patients. Moreover, the effectiveness of using leaflets to educate donors appears to be minimal. One study showed that educational leaflets had a limited effect to reduce serological ineligibility owing to HIV when used in isolation.22 Other educational approaches therefore need to be encouraged.
Clinical screening was also identified as a weak point. This stage is the first step in ensuring safe blood and should be performed by trained and competent personnel, using a pre-defined, validated and standardized questionnaire.23 Clinical screening is even more important for preventing TTM as the sensitivity of the laboratory screening tests used is not high.24 The variation in the questions found in the screening questionnaires shows that there is no standardization among the participating BBs to assess donors in relation to malaria. Studies to validate questions posed to donors with the aim of preventing TTM need to be conducted.
With regard to the selection criteria regarding donors exposed to risk areas, the regulations clearly need improvements. In 1999 Kiesslich et al.25 proposed that API ranges should be used as a parameter for TTM risk assessment. The adopted API grades of risk (low, medium and high risk) have not been created or standardized to achieve this objective. There is no epidemiological data in the literature that enables, a priori, API grades to be established for screening donors and their use needs to be reassessed. We suggest that panel sessions should be held with specialists to define the utility of API as a parameter for donor selection. In the meantime, it is essential that BBs have access to monthly API data so that clinical screening can be performed based on current information and not on information from the previous year, as was the case with the BBs in this study.
Laboratory screening for malaria revealed relevant shortcomings, such as not testing all donors, the absence of EQE for malaria tests and, in the case of the thick blood smear test, an insufficient number of fields were inspected (less than 200) to conclude that the slides were free of Plasmodium.
The thick blood smear test has limited sensitivity to detect asymptomatic infections with low parasitemia.24 It must therefore be used with great caution so as to reduce false negative results as far as possible. Since 2001 the MoH has offered a free EQE program for public BBs that includes serological and immunohematological tests. However, malaria has never been included in this program and its inclusion with regard to thick blood smear testing has shown to be relevant.
Screening for malaria in BBs is a worldwide challenge. In non-endemic countries, in general, the option is made to temporarily exclude donors who have traveled to endemic regions and laboratory tests to directly detect the parasite are not performed. In endemic countries consensus does not exist as to best practices to prevent TTM. Some countries with endemic areas, such as Colombia26 and Ethiopia,27 do not use laboratory screening and undertake risk assessment and selection based exclusively on the epidemiological questionnaire. In Turkey,28 selection is done via a questionnaire and, if donors are found to be ineligible, serological and molecular tests can be used to reduce the period of ineligibility, as recommended by European guidelines. As such, we recommend an evaluation as to the possibility of municipals or micro-regions where native cases of malaria have not be recorded for a long time using the same selection criteria as municipals in non-endemic areas. This change would reduce the costs of laboratory screening for malaria, although there might be a possible increase in clinical ineligibility.
Considering the limitations of thick smears as a screening test in BBs, together with the possibility of reducing the number of BBs obliged to perform laboratory screening for malaria, we suggest that the cost effectiveness of implanting NAT for Plasmodium species in BBs in endemic municipalities or micro-regions should be evaluated.
The weaknesses found in the process of selecting and identifying donors at risk of TTM indicate the importance of a sensitive and structured hemovigilance system. It is important that donors let BBs know if they have acquired malaria or are suspected of having malaria after donation. Whether or not donors take this initiative depends on them knowing that malaria can be transmitted through transfusions, and on effective communication channels with BBs. The inclusion on the SIVEP-Malaria notification form of a question ‘Have you donated blood in the last 30 days?’, and an information flow between the notifying unit and BBs, could identify donations made during the disease incubation period, thereby reducing TTM cases or enabling timely TTM identification and treatment, reducing its lethality. Nevertheless, asymptomatic donors who have malaria continue to be a challenge for BBs and hemovigilance has become an essential mainstay for early TTM case identification.
This study has some methodological limitations worthy of mention. The study's design is cross-sectional and the situation analyzed may have changed following it, possibly as a consequence of the intervention used for this the study. Moreover, the evaluation used a regulation that was revoked during the course of the study. Nevertheless, the alterations contained in the new regulations (ANVISA Resolution RDC 57/2010 and MoH Ordinance 2712/2013) were minimal with regard to screening donors for malaria in endemic areas, such as (i) municipalities where donors live or have traveled was defined as the API assessment area, (ii) definition of ineligibility periods in cases of having traveled to high-risk areas and (iii) permission to use laboratory tests that detect Plasmodium antigens. These alterations do not alter the scores and the classifications obtained in this study. Moreover, all information received was self-reported and the answers were confirmed, whenever possible, by documents. However, as it was an external evaluation, despite the people legally responsible for the establishments agreeing to take part in the study, at times access was not authorized to SOPs and, in these cases, measures were not adopted to confirm the answers via a different means of verification. This situation was rare however (2 cases). As a general rule, the person in charge of the sector was consulted in order to obtain more precise answers regarding the practice in question. If this person was not available, then the person technically responsible for the BB answered the questionnaire (1 case). As such, verification errors may have occurred in some cases. With regard to the scores, we emphasize that the weight used for each question was decided by the researchers based on arbitrary criteria as to the relevance of the question for the potential risk of TTM occurrence. Other scoring systems could have generated different final classification results. Nevertheless, the validation of such scoring and classification systems is limited by the small number of events.
Despite these limitations, the results of the logical framework proposed are considered to have met the objectives of evaluating BB structure and processes and can be used for internal evaluations and correction of activities. They can also be used by VISA in support of its actions, in particular inspections.
Finally, underlining the relevance of this subject, it should be noted that in 2004 the World Health Organization launched the Global Patient Safety Challenge, which includes ‘blood safety’ as one of its pillars. As such, programs are required to be implanted that ensure accessibility of high quality and safe blood to all those who really need it.29 Donor selection and hemovigilance are key elements of this process. Transmission of any disease represents a failure and best practices must be adopted so that patients are not harmed by blood. This includes taking care to ensure the prevention of TTM.
ConclusionThe study provides evidence that adherence to TTM prevention regulations was neglected by the participating BBs. None of the BBs obtained an ‘adequate’ classification, either for the overall classification or for the clinical or laboratory screening components. BB professionals, MoH and VISA managers need to pay greater attention to this matter so that the safety procedures required by the standards are complied with, in particular the preparation of and compliance with SOPs for clinical and laboratory screening of malaria, the inclusion of malaria detection EQE and the implementation of the hemovigilance system. In addition, we believe that these regulations, even after the 2010 and 2013 revisions, can be further improved.
Conflicts of interestThe authors declare no conflicts of interest.
To the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Agência Nacional de Vigilância Sanitária (ANVISA), from Brazil, for funding this study, as well as to the blood banks taking part in the study for allowing access to donor data and facilities.