The use of peripheral hematopoietic progenitor cells (HPCs) is the cell choice in autologous transplantation. The classic dose of granulocyte-colony stimulating factor (G-CSF) for mobilization is a single daily dose of 10μg/kg of patient body weight. There is a theory that higher doses of granulocyte-colony stimulating factor applied twice daily could increase the number of CD34+ cells collected in fewer leukapheresis procedures.
ObjectiveThe aim of this study was to compare a fractionated dose of 15μg G-CSF/kg of body weight and the conventional dose of granulocyte-colony stimulating factor in respect to the number of leukapheresis procedures required to achieve a minimum collection of 3×106 CD34+ cells/kg body weight.
MethodsPatients were divided into two groups: Group 10 – patients who received a single daily dose of 10μg G-CSF/kg body weight and Group 15 – patients who received a fractioned dose of 15μg G-CSF/kg body weight daily. The leukapheresis procedure was carried out in an automated cell separator. The autologous transplantation was carried out when a minimum number of 3×106 CD34+ cells/kg body weight was achieved.
ResultsGroup 10 comprised 39 patients and Group 15 comprised 26 patients. A total of 146 apheresis procedures were performed: 110 (75.3%) for Group 10 and 36 (24.7%) for Group 15. For Group 10, a median of three (range: 1–7) leukapheresis procedures and a mean of 8.89×106 CD34+ cells/kg body weight (±9.59) were collected whereas for Group 15 the corresponding values were one (range: 1–3) and 5.29×106 cells/kg body weight (±4.95). A statistically significant difference was found in relation to the number of apheresis procedures (p-value <0.0001).
ConclusionsTo collect a minimum target of 3×106 CD34+ cells/kg body weight, the administration of a fractionated dose of 15μg G-CSF/kg body weight significantly decreased the number of leukapheresis procedures performed.
The autologous transplantation of peripheral hematopoietic progenitor cells (HPCs) is widely used to treat solid tumors and lymphomas in children, adolescents and young adults.1–3 Since the end of the 1980s the use of leukapheresis has been the most used method to harvest cells for autologous transplantation as it is considered to be effective and safe, promoting a faster hematopoietic recovery and posing less risk to the patient (no general anesthesia and no anemia). As a consequence, this method is associated with lower cost and there is less risk of contamination of the graft by neoplastic cells compared to bone marrow.4 According to the Center for International Blood and Marrow Transplant Research (CIBMTR),5 around 32,000 transplants were carried out in the last decade with, in most cases, the cell source being peripheral HPCs.5 In 91% of cases where a bone marrow transplant is needed in the child population of the US, peripheral HPCs are harvested with the most common reasons for treatment being lymphomas and solid tumors.5
Myelosuppressive chemotherapy or high doses of chemotherapy in association with granulocyte-colony stimulating factor (G-CSF) has been successfully used in the mobilization of peripheral HPCs with a reduction of contamination by neoplastic cells.6,7 These treatments are safe and well tolerated with a large capacity for mobilization and action against neoplastic cells. Cyclophosphamide (CY), alone or in combination with other agents, is the most commonly used chemotherapy drug8 although other regimens such as the ifosfamide, carboplatin and etoposide (ICE) and dexamethasone, adriamycin and cisplatin regimens (DHAP) are also employed.9–11 However, the most effective regimen, with the most suitable intensity of mobilization, remains to be defined.4,12
G-CSF is the most potent cytokine available4 and the one most commonly used for the mobilization of peripheral HPCs.5 G-CSF has low toxicity and is well tolerated. The most common side effect is mild bone pain, beginning after two or three applications,13 however, few patients need to reduce the dose or discontinue treatment.4 In autologous transplantation, the classic G-CSF dose for mobilization is 10μg/kg of patient body weight (bw) via subcutaneous administration once a day.12,14 Some studies have shown that larger doses of G-CSF and fractionated doses given in two daily applications increase the number of CD34+ cells collected with a lower number of leukapheresis procedures.15,16 Moreover, in patients whose mobilization failed with the conventional dose of G-CSF, an increase in the dose to 12.5–50μg/kg bw/day can be successful.6,17 Subcutaneous G-CSF administration achieves a maximum serum level within 2–8h after application with a half-life of 3–4h.18 Thus, a single daily application may not be optimal.18
The recognized method of harvesting peripheral HPCs is large-volume leukapheresis (LVL).5 With current technology, leukapheresis can be carried out in very young children with low weights (<10kg), allowing a sufficient number of CD34+ cells to be collected for complete bone marrow recovery after high doses of safely applied chemotherapy.18–20
The determination of the time at which to begin leukapheresis is based on several factors, such as the kinetics of leukocyte recovery after myelosuppressive chemotherapy, the peripheral platelet count, the absolute number of leukocytes in the peripheral blood and the concentration of circulating peripheral CD34+ cells.21–23 The most commonly applied criteria are an absolute leukocyte count ≥1×103 cells/μL and a CD34+ cell concentration ≥10 cells/μL.22,23
The increased circulation of peripheral CD34+ cells after mobilization lasts a short time and thus it is fundamental that apheresis is carried out during this period in order to collect a sufficient number of CD34+ cells. The peak in CD34+ cells is reached after the leukocyte nadir in the recovery from aplasia caused by myelosuppressive chemotherapy.24 The moment at which there is a sufficient quantity of CD34+ cells for collection by apheresis is when the peripheral leukocyte count reaches ≥1×103 cells/μL after recovery from the nadir.25 Consequently, it is proposed that the peripheral HSC collection should begin when the total leukocyte count reaches ≥1×103 cells/μL after the nadir and the concentration of CD34+ cells in peripheral blood is ≥10 cells/μL.22,23 One of the best indicators of hematopoietic recovery in autologous transplantation is the amount of infused CD34+ cells/kg bw.16 The minimum values for the acceptable number of CD34+ cells to achieve a fast, safe and effective engraftment after autologous transplantation is between 2 and 5×106/kg bw.4,26,27
In order to obtain the ideal number of CD34+ cells collected for autologous transplantation in children, this study investigates whether the use of G-CSF at a fractionated dose of 15μg/kg bw would reduce the number of leukapheresis procedures required to achieve a minimum number of CD34+ cells of 3×106/kg bw and compares the results with those obtained using the conventional single dose of 10μg/kg bw G-CSF.
MethodsThis ambispective study was carried out at a single institution. The eligibility criteria for participation in the study were: under 24-year-old patients recommended for autologous transplantation diagnosed with solid tumors or lymphomas, and treated at the Centro Infantil Boldrini (Boldrini Children's Center) with up to four chemotherapy treatment regimens, and mobilized with chemotherapy and G-CSF. The study was approved by the appropriate ethics committee and informed consent forms were signed by the patients or their guardians.
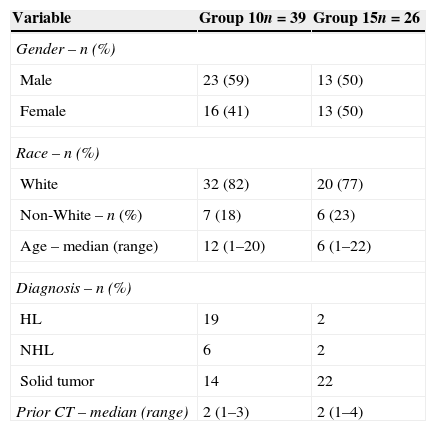
The patients were divided into two groups: Group 10 was comprised of patients who received a single daily dose of 10μg G-CSF/kg bw between December 1998 and December 2008 and Group 15 was comprised of patients who received a fractionated daily dose (defined below in the section on G-CSF) of 15μg G-CSF/kg bw between August 2010 and April 2013. The patient characteristics are detailed in Table 1.
Characteristics of patients in each group.
| Variable | Group 10n=39 | Group 15n=26 |
|---|---|---|
| Gender – n (%) | ||
| Male | 23 (59) | 13 (50) |
| Female | 16 (41) | 13 (50) |
| Race – n (%) | ||
| White | 32 (82) | 20 (77) |
| Non-White – n (%) | 7 (18) | 6 (23) |
| Age – median (range) | 12 (1–20) | 6 (1–22) |
| Diagnosis – n (%) | ||
| HL | 19 | 2 |
| NHL | 6 | 2 |
| Solid tumor | 14 | 22 |
| Prior CT – median (range) | 2 (1–3) | 2 (1–4) |
HL: Hodgkin lymphoma; NHL: Non-Hodgkin lymphoma; Solid tumor: Germ cell tumor, Neuroblastoma, Ewing sarcoma and Medulloblastoma; CT: Chemotherapy treatment
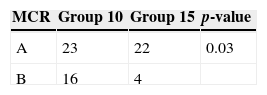
The mobilization chemotherapy regimens (MCR) administered using cyclophosphamide (Regimen A) included CY (cyclophosphamide 4g/m2/day×1 day), Topo/CY (topotecan 0.75mg/m2/day×5 days and cyclophosphamide 250mg/m2/day×5 days), Cy/Vp-16 (cyclophosphamide 4–7g/m2/day×1 day and etoposide 4g/m2/day×1 day), Cy/MTX/VP-16 (cyclophosphamide 4–7g/m2/day×1 day, methotrexate 8g/m2/day×1 day and etoposide 4g/m2/day×1 day) and Cy/Adria (cyclophosphamide 2.4g/m2/day×2 days and adriamycin 20mg/m2/day×3 days) and those without cyclophosphamide (Regimen B) were ICE (ifosfamide 3g/m2/day×3 days, carboplatin 500mg/m2/day×2 days and etoposide 150mg/m2/day×3 days), TIP (taxol 175mg/m2/day×1 day, ifosfamide 1.2g/m2/day×5 days and cisplatin 20mg/m2/day×5 days), IFO/Vp-16 (ifosfamide 2.5g/m2/day×5 days, cisplatin 40mg/m2/day×4 days, doxorubicin 10mg/m2/day×4 days and etoposide 125mg/m2/day×4 days), DHAP (dexamethasone 40mg/day×4 days, cytarabine: 400mg/m2/day×1 day and cisplatin 100mg/m2/day×1 day).
Granulocyte-colony stimulating factorG-CSF (Granulokine®; Roche, SP, Brazil/Leucin®; Bergamo, SP, Brazil/Filgrastine®; Blausiegel, SP, Brazil) is the most potent cytokine available and is the most used in the mobilization of peripheral HPCs5,6 as it has synergistic actions with other growth factors which induce mobilization. In this study the administration of G-CSF was started one day after the end of the MCR. The G-CSF was administered subcutaneously at 6:00a.m. as a single dose for Group 10 (10μg/kg bw) and as two doses for Group 15, 10μg/kg bw at 6:00a.m. and 5μg/kg bw at 6:00p.m. This was continued daily for both groups until successful collection of the minimum amount of 3×106 CD34+ cells/kg bw or until the characterization of lack of mobilization was determined by an unsuccessful collection.
Vascular accessA non-completely implantable two-way central venous catheter was introduced into all patients in order to carry out the LVL.
Large-volume leukapheresisLeukapheresis was carried out in an automatic continuous flow cell separator with the anticoagulant citrate dextrose solution (ACD-A – CS3000 plus®; Cobe-spectra®; Com.tec®). The first LVL procedure for Group 15 was carried out after the MCR with ≥1×103 cells/μL of leukocytes and ≥10 cells/μL of CD34+ in the peripheral blood and for Group 10 with ≥1×103 cells/μL of leukocytes. Patients who weighed <20kg received priming with irradiated and filtered packed red blood cells (PRBCs) (20mL/kg bw). During the LVL all patients received intravenous (IV) replacement of calcium, sodium, potassium and magnesium and were under continuous monitoring. Four blood volumes were processed per leukapheresis procedure and if symptoms and/or signs of hypocalcemia related to the anticoagulant used in the cell separator system were observed, a dose of 0.2mL/kg bw of 10% calcium gluconate was administered intravenously. All patients who did not reach the minimum number of CD34+ cells (3×106/kg bw) in the first leukapheresis procedure were submitted to another procedure on the following day regardless of the number of leukocytes and CD34+ cells in the peripheral blood. A limit of two more leukapheresis procedures was set for Group 15 but with no limit in the number of procedures for Group 10.
Complete blood count after large-volume leukapheresisA complete blood count was obtained after each LVL procedure. A prophylactic transfusion was carried out with an irradiated and filtered platelet concentrate (PC) if the number of platelets in the peripheral blood was <10×103 cells/μL and irradiated and filtered PRBC transfusion if the hemoglobin (Hb) was <8.0g/dL.
Peripheral hematopoietic progenitor cell analysisThe nucleate cell (NC) count for the leukapheresis product was carried out using the automatic counter used to obtain the complete blood count and the percentage of CD34+ cells was determined by flow cytometry using the standard Sutherland Technique.28 To carry out the autologous transplantation the number of CD34+ cells was defined as 3×106/kg bw.
Statistical analysisA descriptive analysis of all variables involved was carried out and then the Chi squared or Fisher exact tests were applied as appropriate to the categorical variables while the Student t-test was applied to the continuous variables to compare the means of Groups 10 and 15. Statistical significance was set for p-values <0.05. The Statistical Package for the Social Sciences (SPSS) version 21.0 was use in all analyses.
ResultsFifty-three patients received 10μg G-CSF/kg bw between December 1998 and December 2008 (Group 10). Fourteen patients were excluded from the analysis: eight mobilized only with G-CSF, one was 29 years of age and mobilization failed in five cases. Between August 2010 and April 2013, 35 patients received a fractionated dose of 15μg G-CSF/kg bw daily (Group 15) with nine being excluded from the analysis due to mobilization failure.
Of the 65 patients analyzed, 55.4% were male, the median age was ten years (1–22), 80% were white and 55.4% were diagnosed with solid tumors. The median number of pre-mobilization chemotherapy cycles received was two for both groups; varying from one to three for Group 10 and one to four for Group 15, with no statistically significant difference, and with no influence on the number of CD34+ cells collected.
With regard to the MCR, Regimen A was used in 23/39 (59%) of cases of Group 10 and 22/26 (84%) of cases of Group 15 resulting in a statistically significant difference (p-value=0.03) (Table 2). However, no difference in the leukocyte recovery time (≥1×103 cells/μL) was observed between the two regimens and the MCR did not influence the number of leukapheresis procedures per group and no patients had MCR-related complications.
Regimen A: CY (cyclophosphamide 4g/m2/day×1 day), Topo/CY (topotecan 0.75mg/m2/day×5 days and cyclophosphamide 250mg/m2/day×5 days), Cy/Vp-16 (cyclophosphamide 4–7g/m2/day×1 day and etoposide 4g/m2/day×1 day), Cy/MTX/Vp-16 (cyclophosphamide 4–7g/m2/day×1 day, methotrexate 8g/m2/day×1 day and etoposide 4g/m2/day×1 day) and Cy/Adria (cyclophosphamide 2.4g/m2/day×2 days and adriamycin 20mg/m2/day×3 days).
Regimen B: ICE (ifosfamide 3g/m2/day×3 days, carboplatin 500mg/m2/day×2 days and etoposide 150mg/m2/day×3 days), TIP (taxol 175mg/m2/day×1 day, ifosfamide 1.2g/m2/day×5 days and cisplatin 20mg/m2/day×5 days/), IFO/Vp-16 (ifosfamide 2.5g/m2/day×5 days, cisplatin 40mg/m2/day×4 days, doxorubicin 10mg/m2/day×4 days and etoposide 125mg/m2/day×4 days), DHAP (dexamethasone 40mg/day×4 days, cytarabine: 400mg/m2/day×1 day and cisplatin 100mg/m2/day×1 day).
All patients showed good tolerance to G-CSF administration; no applications were suspended or reduced in either group and only five (13%) patients of Group 10 and two (7%) patients of Group 15 reported mild bone pain.
The median number of pre-leukapheresis leukocytes was 14.4×103 cells/μL (range: 0.9–50.3) for Group 10 and 22.3×103 cells/μL (range: 3.0–73.9) for Group 15. The median concentration of peripheral CD34+ cells was 27.9 per μL (range: 1.10–135.0) for Group 10 and 29.5 cells per μL (range: 8.0–90.0) for Group 15, with no statistically significant difference between values. No patients had complications during the leukapheresis procedure in either group; no hypovolemia was observed in children who weighed <20kg and only two (5%) patients in Group 10 reported mild paresthesia related to the use of the anticoagulant. Thirty-two patients, 15 (38%) from Group 10 and 17 (65%) from Group 15, had platelet counts <50×103 cells/μL during the pre-leukapheresis period. However, no type of bleeding occurred and only one (3%) patient of Group 15 received post-leukapheresis irradiated and filtered PC. None of the patients from either group received a transfusion of irradiated and filtered PRBCs after leukapheresis. Three (7%) cases in Group 10 and 11 (42%) cases in Group 15 received priming of irradiated and filtered PRBCs during the leukapheresis procedure.
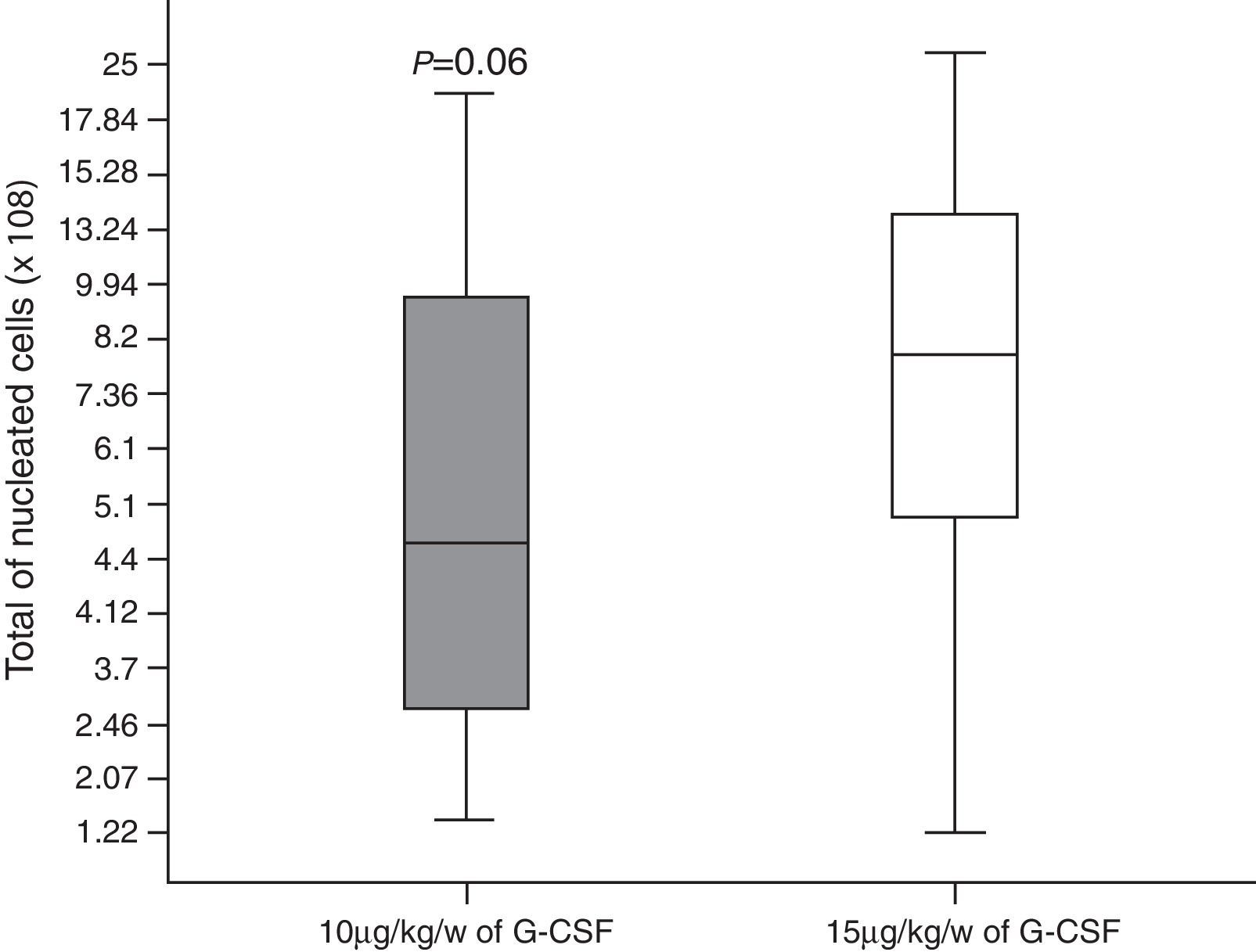
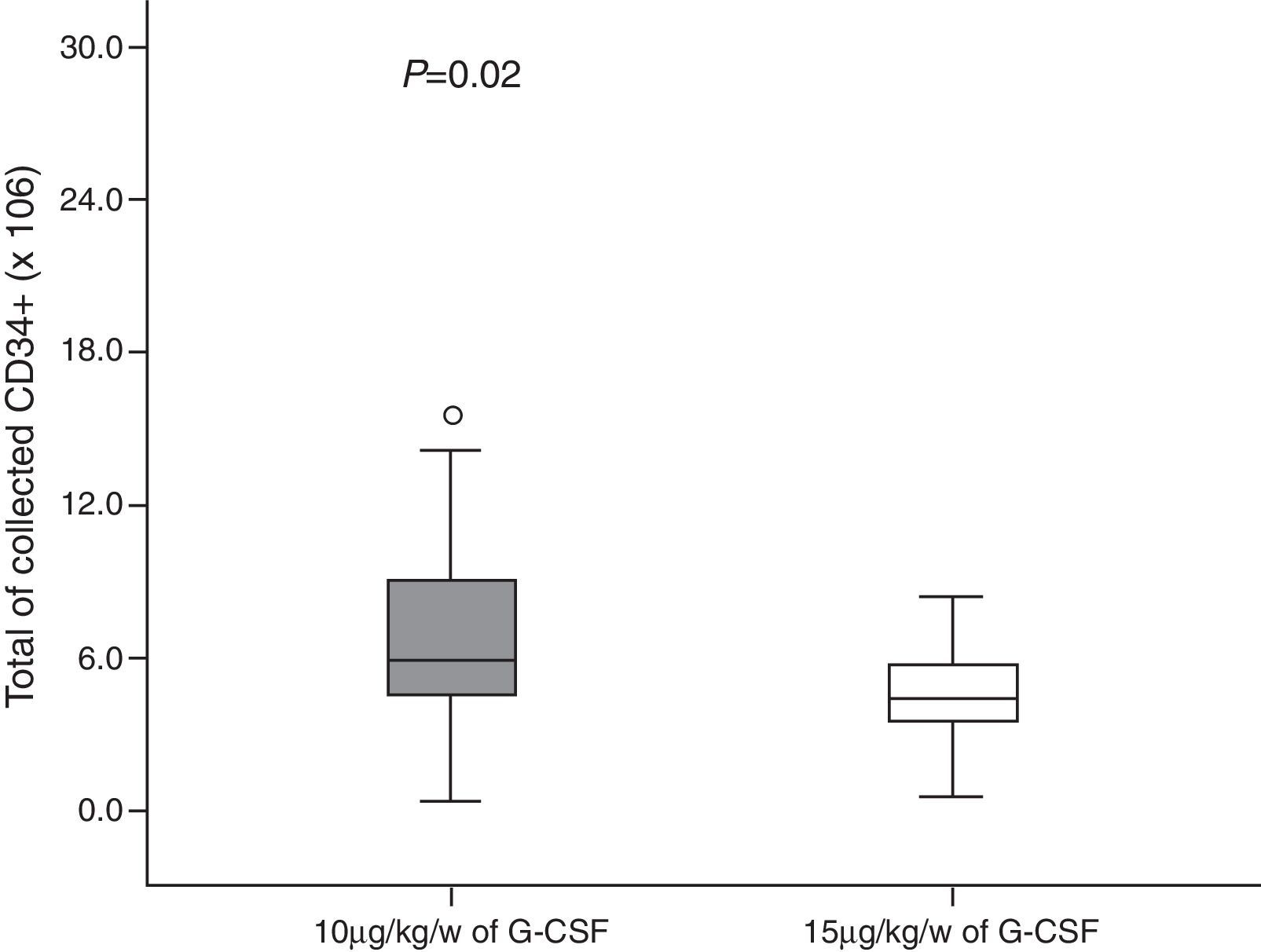
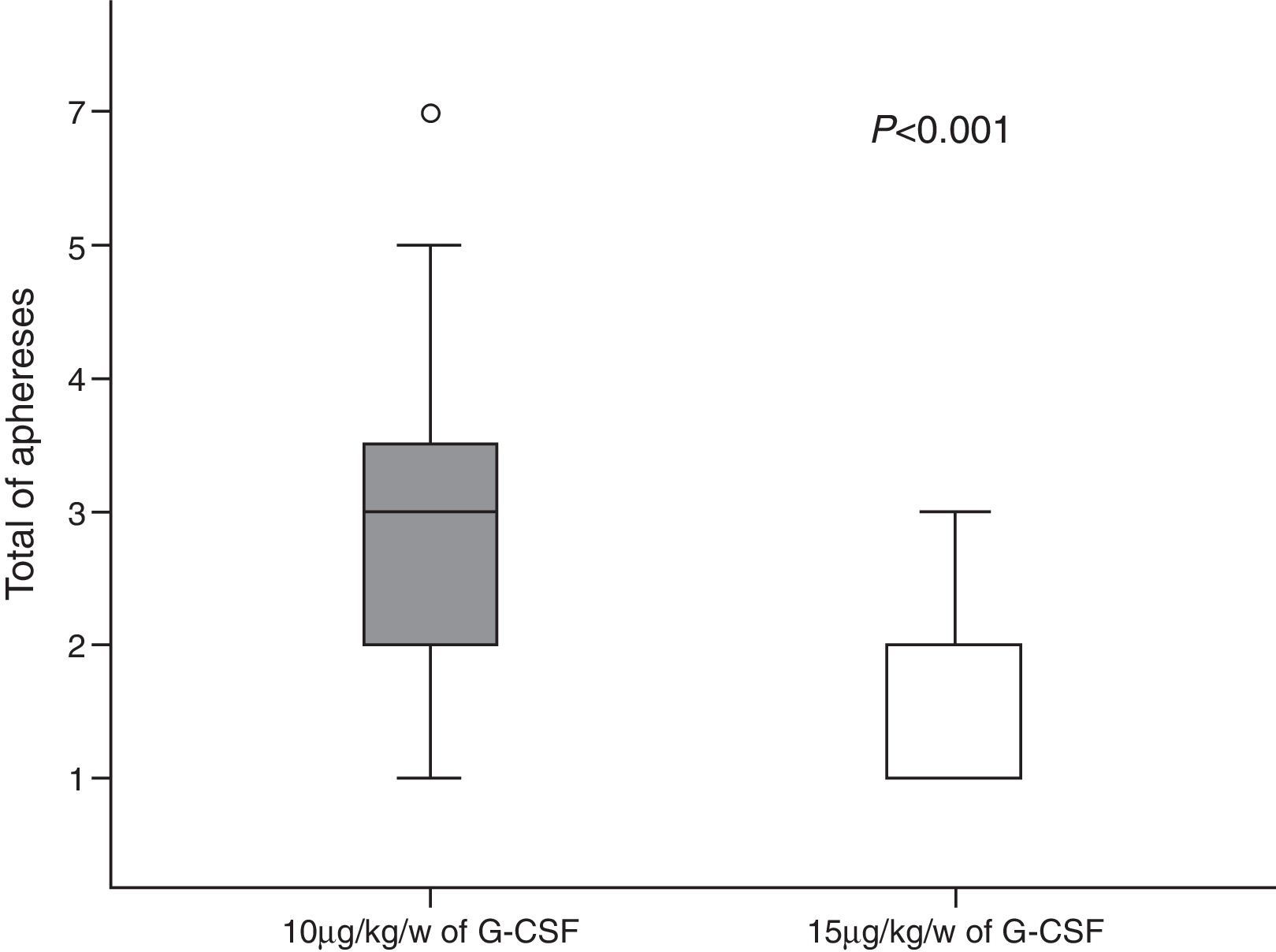
A total of 146 leukapheresis procedures were carried out, 110 in Group 10 and 36 in Group 15, with an average duration of 4h (±2); the second leukapheresis procedure of one patient in Group 15 was interrupted half-way through the procedure due to malfunctioning of the cell separator; there was malfunctioning of the catheter in four patients. Moreover, one patient of Group 10 and one of Group 15 had prolonged LVL times and it was not possible to collect the minimum number of CD34+ cells in two cases of Group 15. A median of three (range: 1–7) leukapheresis procedures were performed for Group 10 with medians of 7.22×108/kg bw (range: 1.28–20.70) and 8.89×106/kg bw (range: 0.3–45) of nucleated cells (NCs) and CD34+ cells, respectively being collected. In Group 15 there was a median of one leukapheresis procedure (range: 1–3) and medians of 10.17×108/kg bw NCs (range: 1.22–37.0) and 5.29×106 CD34+ cells/kg bw (range: 0.6–27.8) were collected (Figures 1 and 2). The median number of days of leukocyte recovery was eight (range: 0–18) for Group 10 and ten (range: 2–13) for Group 15 (p-value=0.15). Thus, statistically significant differences between groups were observed for the number of leukapheresis procedures (p-value <0.0001) (Figure 3) and the number of CD34+ cells collected (p-value=0.02), while the total NC count resulted in a favorable tendency toward Group 15 (p-value=0.06).
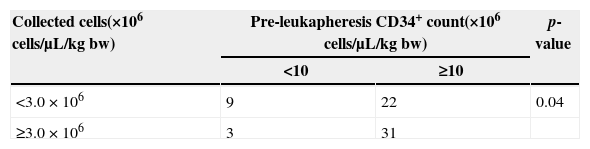
A statistically significant distribution was observed on comparing groups in respect to pre-apheresis peripheral CD34+ cells (≥10 or <10 cells/μL) and the number of CD34+ cells collected (≥3.0 or <3.0×106 cells/μL) (Table 3).
DiscussionSome authors23,29 suggest that the number of pre-MCR received negatively influences the number of CD34+ cells collected, which was not observed in this study. The MCR used in this study did not cause complications, even in patients taking high doses of cyclophosphamide (4–7g/m2). It is known that each patient responds in a distinct way to mobilization and that other parameters may have a negative effect, such as the time between the diagnosis and harvest, previous irradiation, thrombocytopenia at the time of mobilization and many other factors cited in the literature.5,23,28,29 Consequently, in this study it was not possible to define the best mobilization chemotherapy regimen as is reported in the literature.4,12
The administration of G-CSF was well tolerated as the only side effect reported by the patients was mild bone pain in 10% of the cases which is the side effect most commonly reported.13 Priming with irradiated and filtered PRBCs of children weighing below 20kg allowed the LVL procedure to be safely carried out without complications. In the two cases of mild anticoagulant-related paresthesia, the condition was reverted using intravenous calcium gluconate. Problems related to the non-completely implanted two-way catheter were few with only around 6% of the catheters malfunctioning, a lower rate than reported in the literature.22,29
With the exception of one leukapheresis procedure (1/146), which was interrupted half way through, the cell separators functioned acceptably and there was no need for technical adjustment in the other leukapheresis procedures. The time of leukapheresis was well tolerated including by under 5-year-old children as there were no complaints from patients who experienced prolonged processing times; these patients did not show signs or symptoms of anticoagulant-related hypocalcemia.
The absolute peripheral leukocyte count (≥1×cells/μL) and CD34+ cell concentration (≥10 cells/μL) were found to be good parameters to start leukapheresis, in particular the peripheral CD34+ cell concentration (≥10 cells/μL), even though this parameter cannot be used for all patients.
In Group 15, the fractionated application of 15μg G-CSF/kg bw decreased the number of leukapheresis procedures required to obtain the minimum number of CD34+ cells established, and although a maximum of three leukapheresis procedures was defined for this group, the median was one procedure. A factor which may appear to be a limitation in this study, as the maximum number of leukapheresis procedures for this group was established at three a priori, and which was in fact observed, was that the fractionated dose led to the minimum number of CD34+ cells being achieved without the need for further collections, that is, regardless of the study design, this group did not require further leukapheresis procedures. Only one patient underwent three leukapheresis procedures, while around 65% achieved the target after only one procedure. Consequently, these results indicate that the efficiency of the fractionated dose needs to be evaluated in greater detail.
ConclusionsThis study demonstrated that the mobilization and collection of peripheral HPCs in children is viable and safe. The absolute peripheral leukocyte count (≥1×103 cells/μL) and CD34+ cell concentration (≥ 10 cells/μL), when used together, are good parameters to indicate the start of leukapheresis. The fractionated application of 15μg G-CSF/kg bw significantly reduced the number of leukapheresis procedures needed to collect a minimum of 3×106 cells/kg bw CD34+ cells.
Conflicts of interestThe authors declare no conflicts of interest