The hemagglutination reaction, when applied to immunohematology, whether positive or negative, suggests the presence or absence of Red Blood Cell (RBC) antigens, respectively. The simultaneous occurrence of positive and negative agglutination in the same sample, called Mixed Field (MF), results from distinct antigenic phenotypic changes in RBC.1
Different factors may contribute to MF. Among them are transfusions and allogeneic transplants of non-identical hematopoietic stem cells (HSC), chimerism or mosaicism of hematopoietic cells, and mutations that weaken RBC expression antigens.1 The occurrence of MF resulting from chimerism or mosaicism is not always explained by Mendelian inheritance.2
Post-transfusion or post-transplant of non-identical HSC MF is acquired and transient. It is influenced by the allogeneic transfusion demand and the transplant transition, ranging from the grafting of transplanted HSC to the complete replacement of autologous RBC by allogeneic ones.1 On the other hand, the MF saw in hematopoietic mosaicism may result from mutations affecting the gene regulation in the steps prior to RBC differentiation. It can affect different proportions of RBC and remains for the whole life.2
The MF resulting from hematopoietic mosaicism involving only the ABO system is uncommon in healthy individuals. Two reports of hematopoietic mosaicism showed MF with different RBC populations in blood donors. In one of them, Beattie and colleagues reported a healthy male Negro blood donor carrying an unequal proportion (10:1) of A and B RBC, respectively.3 In another, Borley and colleagues found a proportion of 40% of A1 and 60% A1B RBC in a Caucasian female blood donor.4 These reports demonstrate that hematopoietic mosaicism is one of the causes of MF associated with the ABO system and that, although rare, it occurs in healthy individuals. In this study, we describe the strategies used to resolve a case of a male blood donor with MF in ABO phenotyping resulting from presumed hematopoietic mosaicism.
Material and methodEthical considerationsThe Research Ethics Committee approved this study of FAMERP (CAAE 34163114.6.0000.5415). All analysed individuals received information about the research objectives and signed an informed consent form.
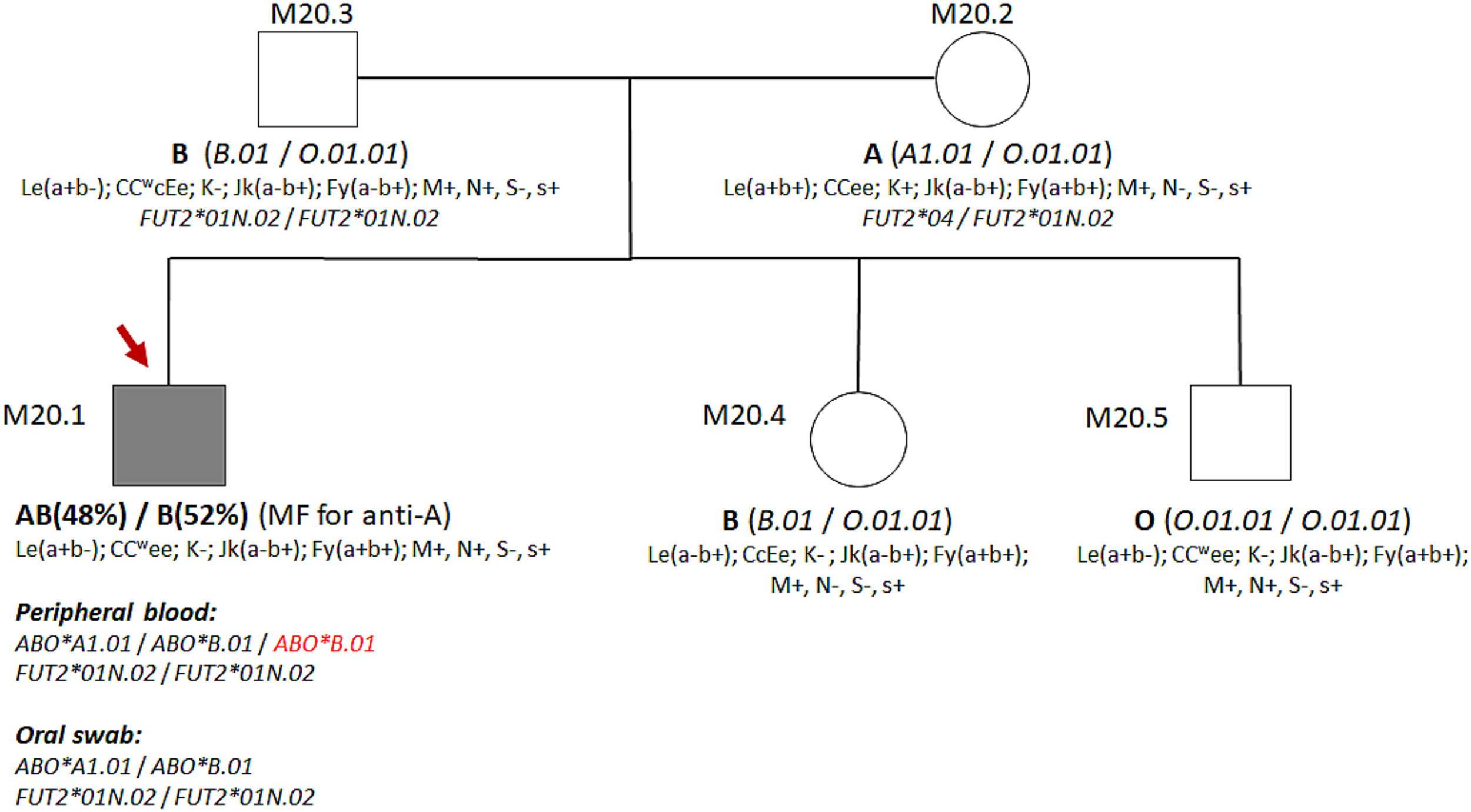
Description of the caseSamples from a 24-year-old male blood donor showing an MF in the forward ABO phenotyping were referred to our service. Their RBCs presented MF reaction with anti-A antiserum but not with anti-B and anti-A,B antisera. The reverse phenotyping suggested he was an AB phenotype. Analyses with anti-A1 lectin showed a reaction in MF equal to one observed with anti-A. The blood donor declared not to have twin brothers and was never transfused or transplanted. Peripheral blood samples, genomic DNA, saliva, and oral swab samples from him and his family members were analyzed as needed for the investigation. Phenotyping for 17 additional RBC antigens did not reveal MF.
Serological investigations of RBCForward and reverse ABO phenotyping was performed in column agglutination technology (CAT) (DiaMed - Latin America, Brazil) and tubes using commercial antisera anti-A (clone: 9113D10), anti-B (clone: 9621A8), and anti-A,B (clone: 152D12 + 9113D10) (Fresenius-Kabi, Brazil; Lorne Laboratories, UK). Additional tests were also performed with commercial anti-A1 (Dolichos biflorus) and anti-H (Ulex europaeus) lectins in CAT, neutral gel (CAT-NG) (DiaMed - Latin America, Brazil).
The antigens of the Rh systems (D, C, Cw, c, E, e), Kell (K), Kidd (Jka, Jkb), Duffy (Fya, Fyb), MNS (M, N, S, s) were identified using CAT (DiaMed - Latin America, Brazil). Lewis RBC phenotypes were identified in test tubes with commercial anti-Lea (clone: LEA2 and 3643B9) and anti-Leb (clone: LEB2 and GX336) antisera (Lorne Laboratories, USA; Fresenius-Kabi, Brazil).
RBC separation from MFThe RBC from MF were separated according to Miola et al.5 protocol, with adaptations in test tubes with commercial anti-A antiserum.
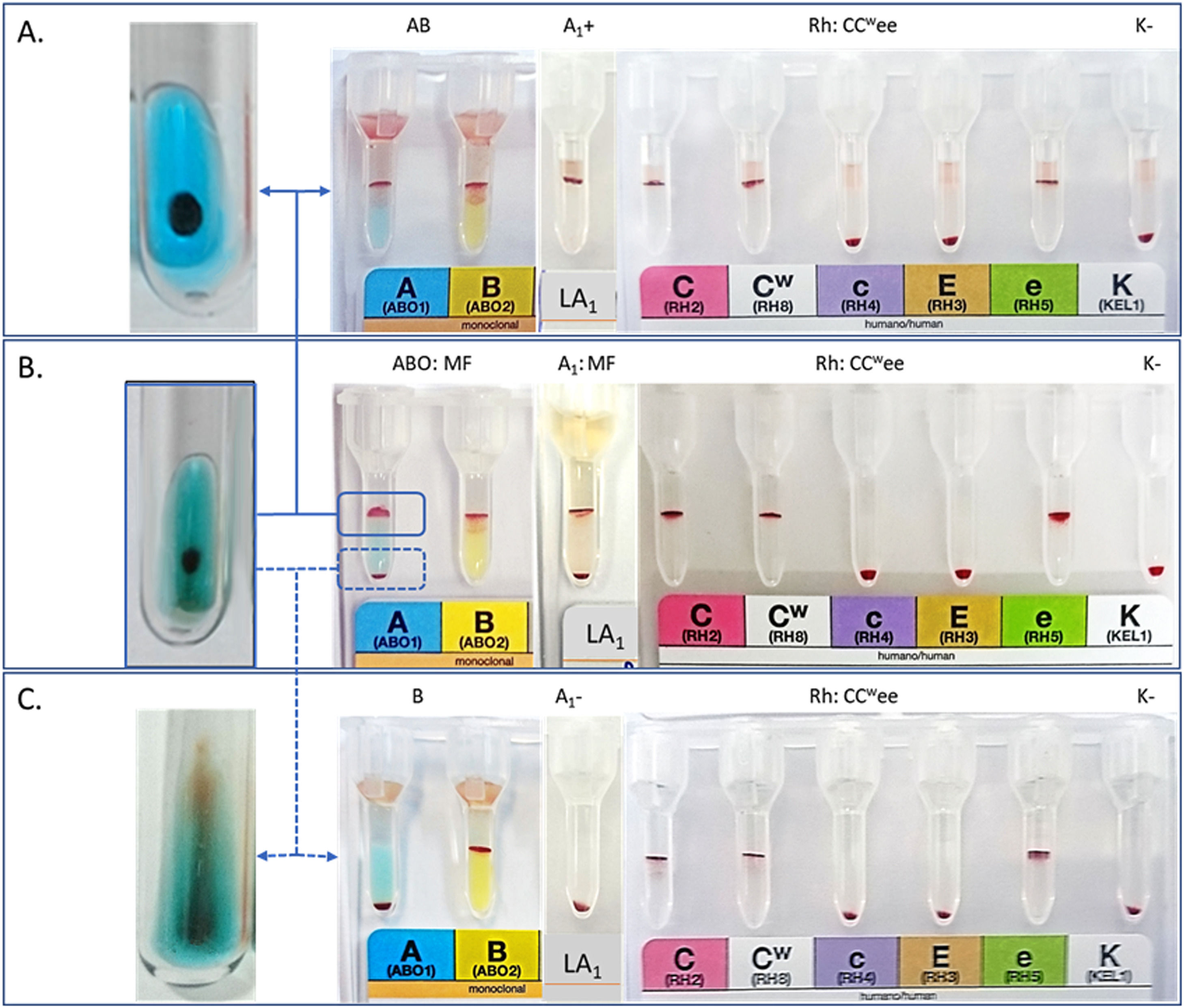
After reacting with anti-A antiserum, non-agglutinated RBCs were removed, transferred to another tube, and washed in an isotonic solution. To remove anti-A antibodies from agglutinated RBCs, they were washed in an isotonic solution and treated with 0.2 M Dithiothreitol (DTT). RBC percentages in the two phases of MF were performed in a cell counter (XN 2000 – Sysmex Europe GmbH, Bornbarch, Germany). The suspensions of both RBCs were adjusted according to the method used (CAT or test tube) (Figure 1). The unreactive RBCs in the MF were analyzed by adsorption and elution with anti-A antiserum (clone: 9113D10) and tested with higher-titers monoclonal antisera (HTMA), anti-A (1024), and anti-A,B (2048), according to the protocol of Miola and collaborators.6
Serological investigations of salivaThe Secretor and non-Secretor phenotypes were determined in saliva samples with the same antisera prior mentioned, according to the protocol by American Association for Blood Banks.1
Molecular investigationsGenomic DNA was extracted from peripheral blood using commercial kits (QIAamp DNA Blood Mini Kit, QIAGEN, Hilden, Germany). Genomic DNA from the oral swab was extracted using a phenol-chloroform method.5 The characterization of the ABO alleles was performed by PCR-RFLP and sequencing from exon 2 to exon 7. The FUT2 (Secretor) genotyping was carried out by sequencing, according to the protocol of Miola and collaborators.5,6
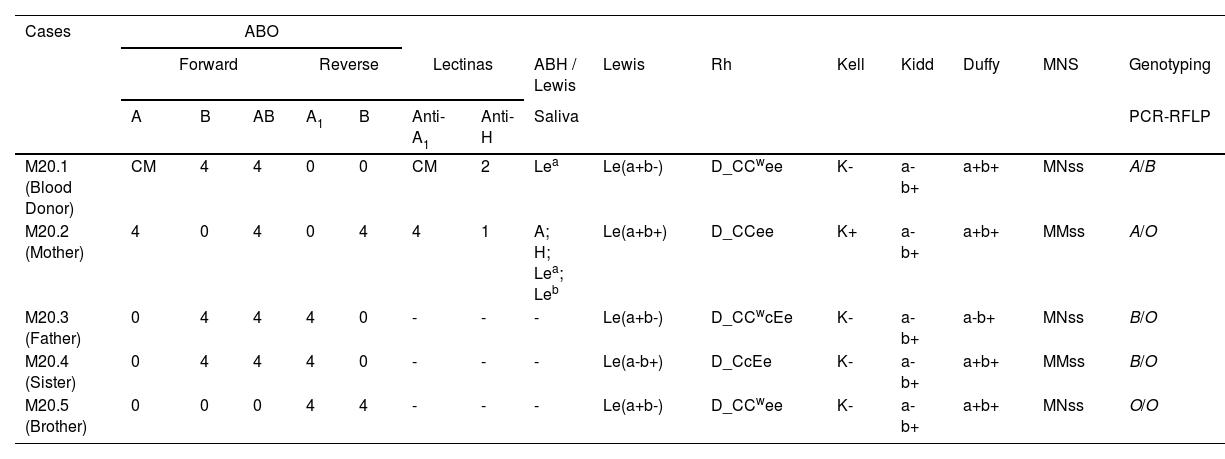
ResultsThe results of the serological and molecular analyses are shown in Table 1 and Figure 3. The reaction in MF was observed only in the forward ABO phenotyping with anti-A monoclonal antiserum and anti-A1 lectin but not in the other identified antigens. Separation of MF reactive and non-reactive RBCs showed that 52% did not have the A antigen but the B antigen, while the remaining 48% had both antigens when phenotyped with anti-A and anti-B antisera (Figure 1). This separation procedure eliminated the MF and allowed to verify that part of the RBCs was type B and part of type AB. The eluate and tests with HTMA of anti-A did not detect the presence of the A antigen in the MF non-reactive RBCs.
Serological and genotyping data from the blood donor and his relatives.
Lewis RBC phenotyping showed that the blood donor is Le(a+b-), and his mother is Le(a+b+). The hemagglutination inhibition test in saliva was in agreement, demonstrating the presence of Lea antigen and A, H, Lea, and Leb antigens, respectively (Table 1). These data indicate that the blood donor has the non-Secretor phenotype.
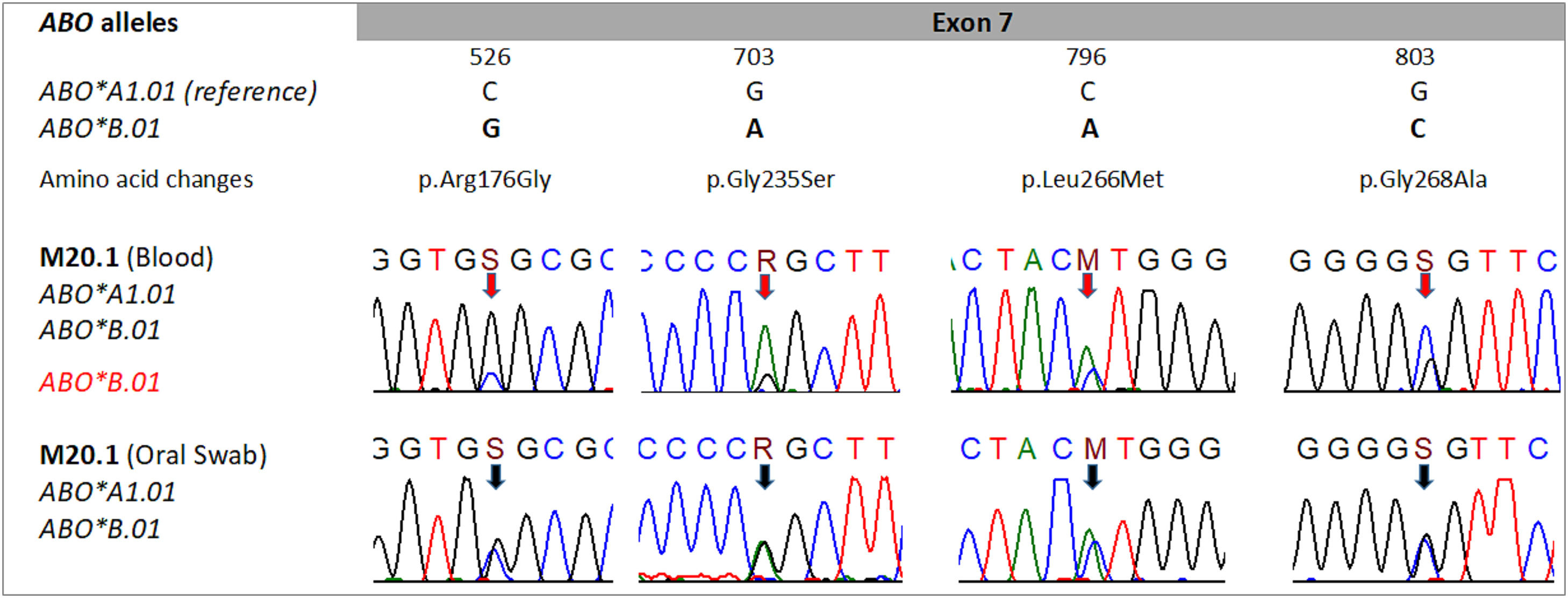
Electropherograms obtained from peripheral blood genomic DNA sequencing revealed distinct signals for alleles A and B at positions 526, 703, 796, and 803 of exon 7 of the ABO gene. These weak signals coincide with the absence of the A antigen in half of the RBCs (52% of the MF). The electropherograms from the sequencing of genomic DNA extracted from the oral swab showed equivalent signals at the mentioned positions, indicating the presence of an A allele and another B allele (Figure 2).
Data from sequencing of the ABO gene. Direct sequencing reveal signals in all nucleotide positions 526, 703, 796, and 803, suggesting heterozygosis in the main alleles ABO*A1.01 and ABO*B.01 in DNA from peripheral blood (red arrows) and DNA from oral swab (black arrows). Distinct signals in the DNA from peripheral blood suggest the presence of mosaicism in the blood donors. The nomenclature S, R, and M follow the IUPAC (International Union of Pure and Applied Chemistry).
Data from ABO genotyping by PCR-RFLP are shown in Table 1. The presence of the O allele was not observed in the blood donor, but his parents and siblings present this allele. The sequencing of the FUT2 gene demonstrated the presence of alleles FUT2*01N.02/FUT2*01N.02 in the blood donor and FUT2*04/FUT2*01N.02 in its mother, being compatible with the non-Secretor and Secretor phenotype, respectively.
DiscussionThis study describes a case with evidence of hematopoietic mosaicism in a type AB blood donor presenting MF in the forward RBC phenotyping with anti-A antiserum. Total agglutination of his RBC with anti-B antisera indicates the presence of B antigen and excludes the presence of O RBCs in the sample. The occurrence of MF with anti-A antiserum demonstrates the presence of the A antigen in part of the RBCs. The protocol for separating reactive and non-reactive RBCs from the MF was crucial to clarify this case.5
Analyses of the RBCs separated from the MF showed a lack of antigen A in 52% and A and B antigens in 48% of the RBCs. Equivalent proportions were also observed in another case in which the authors observed 60% of A1B RBCs and 40% of A1 RBCs.4 Maybe the hematopoietic mosaicism is only characterized by two distinct RBC populations in the ABO system, resulting from alterations in the ABO gene regulation in somatic cells.7
The RBC antigenic profile observed in this study suggests that the blood donor presents mosaicism composed of two populations of RBCs: one of type B and the other of type A1B. This observation is reinforced by the results obtained from eluate analyses and additional ABO phenotyping performed with anti-A antisera containing titers greater than 1024 and with the Anti-A1 lectin (Dolichos biflorus). Phenotyping of other RBC antigens (Rh, Duffy, MNS, Kell, Kidd, Lewis) did not show MF in the blood donor and his relatives, suggesting that the hematopoietic mosaicism generated MF only in the ABO system. These data agree with previous reports demonstrating that hematopoietic mosaicism exclusively affecting the ABO system also occurs in healthy individuals.3,4
In order to reinforce the proposition of hematopoietic mosaicism, we performed molecular analyses by PCR-RFLP and sequencing of the blood donor's genomic DNA. Molecular analysis of exons 6 and 7 of the ABO gene by PCR-RFLP indicated the presence of alleles A and B in both genomic DNA from peripheral blood and oral swab. This result was compatible with the AB genotype and the AB RBC phenotype observed in approximately 48% of the RBC. However, the analysis of the electropherograms obtained from the exon 7 sequencing showed distinct signals in the nucleotides that occupy positions 526, 703, 796 and 803, which differ from the A and B alleles. The peripheral blood genomic DNA sequencing showed divergent signals in these positions, where there was a lower signal for the nucleotides that define the A allele than those that define the B allele. This observation coincides with the absence of the A antigen in approximately 50% of the RBCs. This divergence was not observed in the electropherograms obtained from oral swab genomic DNA sequencing. Differences in signals obtained from peripheral blood genomic DNA sequencing and oral swab were previously reported.5
The presence of identical alleles of the FUT2 gene in the genomic DNA of peripheral blood and oral swab suggests that blood donor mosaicism is restricted to hematopoietic tissue. Furthermore, the FUT2 alleles (FUT2*01N.02/FUT2*01N.02) revealed that the donor is non-Secretory, consistent with the Le(a+b-) phenotype and the presence of Lea antigen in saliva.
The investigation of Secretor and non-Secretor phenotypes and genotypes, although necessary, is not sufficient for the resolution of cases of hematopoietic mosaicism. In Secretory individuals, the A, B, and H antigens will be present in the saliva in the soluble form, contributing to the confirmation of the RBC ABO phenotypes and genotypes. In non-Secretor individuals, only FUT2 genotyping will contribute to the confirmation of RBC phenotypes and genotypes.8 Therefore, in similar cases, FUT2 genotyping is crucial, but the contribution of saliva analysis is dependent on the Secretor status.
This study brings contributions to the resolution of hematopoietic mosaicism cases. The separation and counting of MF reactive and non-reactive RBCs enable antigenic serological characterization of their populations by evaluating each blood group system.5 The careful analysis of the electropherogram allows the verification of distinct signals in the nucleotides that differentiate the A and B alleles, obtained by sequencing of the ABO gene.5 The investigation of Secretor and non-Secretor phenotypes, although necessary, is not always sufficient for the resolution of cases like this in which a single individual presents an unusual serological ABO profile and is incompatible with the Mendelian inheritance model.2 By the authors' knowledge, this is the first description of a hematopoietic mosaicism case involving only the ABO system in a blood donor, reported in Brazil.
Although the results achieved are consistent with hematopoietic mosaicism restricted to the ABO system, this study has limitations. Analyses using FISH (Fluorescent In Situ Hybridization) were not performed as this procedure requires viable cells, especially erythroblasts, for resolution. Obtaining such cells would imply an invasive procedure, generally not indicated for healthy individuals and blood donors. Therefore, it was not possible to reveal whether part of the RBC precursor cells contains two alleles, A and B, and part, only one B allele.
The resolution of this case comprised the procedure of separating the RBCs from the MF with serological analysis and the analysis of the differences in the electropherogram signals of the DNA sequencing of the peripheral blood compared to that obtained from the DNA sequencing the oral swab.
These results suggest that somatic mutations that affect the hematopoietic progenitor lineage (early mosaicism) or myeloid progenitor lineage (late mosaicism) can lead to loss of heterozygosity in about 50% of the affected erythrocyte population (Figure 3).9,10
In conclusion, adequate separation of reactive and non-reactive RBC from MF combined with sequencing of the ABO gene constitutes adequate procedures for resolving rare cases of hematopoietic mosaicism in humans.