ABO, P1PK, LE, H, I, GLOB, and FORS are blood group systems characterized by the expression of carbohydrate antigens in hematopoietic, non-hematopoietic tissues and exocrine secretions. These antigens are synthesized by specific glycosyltransferases adding monosaccharide units to oligosaccharide precursors. The hematopoietic disturbance in some oncohematological diseases affects the expression of ABO, H, and I glycosyltransferases, reducing the synthesis of A and B carbohydrate antigens modifying red blood cell (RBC) phenotypes.1 Acute myeloid leukemia (AML) can induce the transitory loss of RBCs carbohydrate antigens, especially from the ABO system allowing discrepancies in the forward and reverse phenotyping.2 A consistent explanation for this phenomenon is the DNA hypermethylation of the ABO promoter, which underlies the loss of ABO allelic expression in leukemic patients.3,4
These set of events contribute to the expression of natural specific autoantibodies to the ABO, H, and I carbohydrate antigens such as anti-I e anti-IH, anti-i, anti-H, and anti-IA. The majority of these autoantibodies react near 4°C and generally do not interfere in ABO reverse phenotyping. However, in some cases, these cold autoantibodies react at room temperature affecting the correct ABO phenotyping. Anti-I is a common cold antibody that reacts with adult RBCs but can react weakly or not react with cord blood RBCs. This autoantibody sometimes occurs in patients with acute leukemia blood plasma due to the loss of the I antigen and leftover of the i antigen.1 Anti-IH is a cold antibody that reacts with RBCs expressing both I and H antigens. Its reactivity depends on the quantities of H antigen, which vary according to the ABO phenotypes (O>A2>B>A2B>A1>A1B). The anti-IH shows a better reaction with RBCs from adults, especially O, compared to A1.5
Here we report the loss of A antigen at the RBCs and the concomitant presence of cold autoantibodies in two cases of AML patients. Table 1 shows the clinical and laboratory features of cases 1 and 2.
Clinical and laboratory features of 1 and 2 cases.
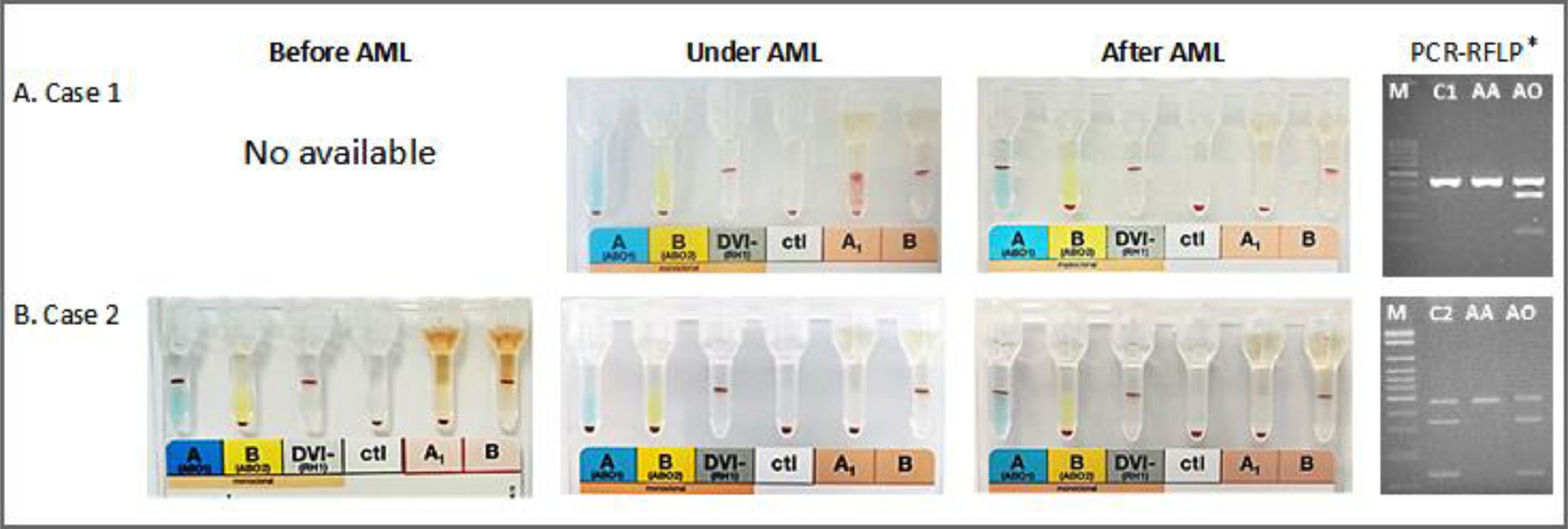
A female patient aged 79, diagnosed with AML in December 2017 (Table 1), needed platelet transfusion due to thrombocytopenia (11,000/mm3). The first pretransfusional test carried out at that time showed that she had O phenotype and weak plasma reactivity with A1 RBC (2+) than B RBC (4+) (Table 2 and Figure 1A). Six RBC samples phenotyped in one month by routine test showed the O phenotype. When tested with high-titer monoclonal antisera (HTMA) anti-A (2048; cell clone 9113D10; Fresenius-Kabi, Brazil; Lorne Laboratories, UK) and anti-A,B (1024; cell clone 9113D10+152D12, Fresenius-Kabi, Brazil; Lorne Laboratories, UK; cell clone ES-15+Birma-1+LB-2, Prothemo, Brazil; Ebram, Brazil), these same samples revealed the presence of A antigen. The reappearance of the A antigen in the RBC was concomitant with reducing the agglutination with anti-H lectin. She presented an anti-I IgM cold autoantibody reacting strongly with A and O adult RBCs but not with O RBCs from cord blood. After six months, she was phenotyped as A by a routine test showing the reappearance of the A antigen. Molecular analysis of exon 6 of the ABO gene showed the AA genotype (Figure 1A).6
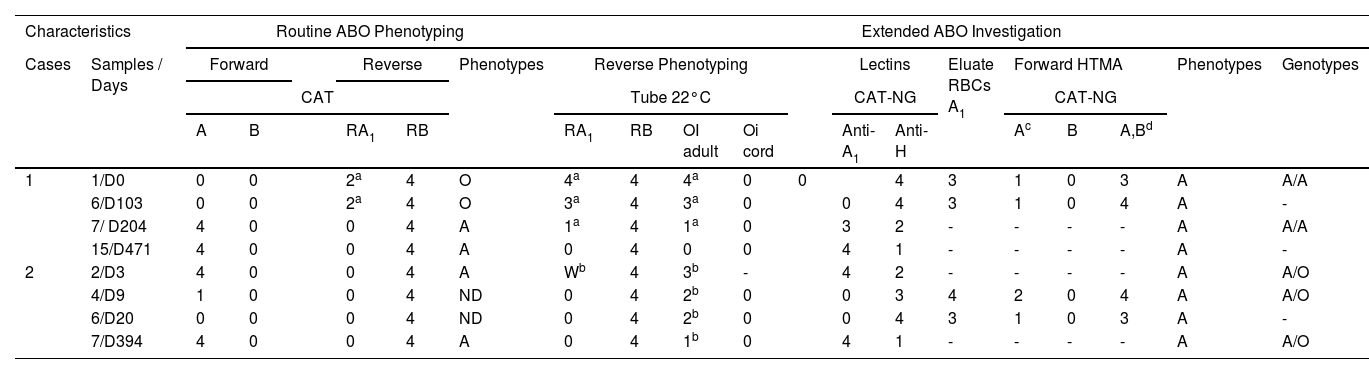
Results of phenotyping and ABO genotyping
| Characteristics | Routine ABO Phenotyping | Extended ABO Investigation | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Samples / Days | Forward | Reverse | Phenotypes | Reverse Phenotyping | Lectins | Eluate RBCs A1 | Forward HTMA | Phenotypes | Genotypes | ||||||||||
| CAT | Tube 22°C | CAT-NG | CAT-NG | |||||||||||||||||
| A | B | RA1 | RB | RA1 | RB | OI adult | Oi cord | Anti-A1 | Anti-H | Ac | B | A,Bd | ||||||||
| 1 | 1/D0 | 0 | 0 | 2a | 4 | O | 4a | 4 | 4a | 0 | 0 | 4 | 3 | 1 | 0 | 3 | A | A/A | ||
| 6/D103 | 0 | 0 | 2a | 4 | O | 3a | 4 | 3a | 0 | 0 | 4 | 3 | 1 | 0 | 4 | A | - | |||
| 7/ D204 | 4 | 0 | 0 | 4 | A | 1a | 4 | 1a | 0 | 3 | 2 | - | - | - | - | A | A/A | |||
| 15/D471 | 4 | 0 | 0 | 4 | A | 0 | 4 | 0 | 0 | 4 | 1 | - | - | - | - | A | - | |||
| 2 | 2/D3 | 4 | 0 | 0 | 4 | A | Wb | 4 | 3b | - | 4 | 2 | - | - | - | - | A | A/O | ||
| 4/D9 | 1 | 0 | 0 | 4 | ND | 0 | 4 | 2b | 0 | 0 | 3 | 4 | 2 | 0 | 4 | A | A/O | |||
| 6/D20 | 0 | 0 | 0 | 4 | ND | 0 | 4 | 2b | 0 | 0 | 4 | 3 | 1 | 0 | 3 | A | - | |||
| 7/D394 | 4 | 0 | 0 | 4 | A | 0 | 4 | 1b | 0 | 4 | 1 | - | - | - | - | A | A/O | |||
CAT: Column Agglutination Technology; CAT-NG: Column Agglutination Technology – Neutral Gel; RBCs: Red Blood Cells; HTMA: High-Titers Monoclonal Antisera; ND: Not Determined; D: Day.
A female patient aged 56 with a history of breast cancer in 2013 and treated with chemotherapy and radiation therapy was diagnosed with therapy-related myelodysplastic syndrome (tMDS) in 2019 and overt therapy-related AML (tAML) in May 2020 (Table 1), undergoing treatment with 5-azacitidine with no induction, presented A blood type according to the forward and reverse phenotyping. In 20 days of follow-up, routine tests showed reduction and loss of the A antigen. Tests with the same HTMA anti-A (2048) and anti-A,B (1024) used in the investigation of case 1, revealed A antigen. The loss of the A antigen in the RBC, detected after tAML, was concomitant with increased agglutination with anti-H lectin. She presented an anti-IH IgM cold autoantibody reacting with O adult RBCs but not with O RBCs from cord blood and a weak reaction with A RBCs (Table 2). Molecular analysis of exon 6 of the ABO gene showed the AO genotype (Figure 1B).6
DiscussionThis report of two cases of AML shows the loss and the reappearance of A antigen in the RBCs. The first patient presented an O blood type at admittance but recovered her original A blood type after complete remission of the disease. The second patient was phenotyped as A blood type, but she remained O blood type after losing the A antigen. She recovered her original A blood type after the partial remission of the disease. The loss of ABO carbohydrate antigens from RBCs is a known phenomenon in myeloid malignancies.7 DNA methylation affects the gene regulation of the ABO gene underlying the loss of ABO allelic expression in a significant proportion of leukemia patients.3,4 Both patients reported here recovered their original A blood type after treatment with hypomethylating drugs.
We applied a modified protocol using high titers of anti-A and anti-A,B antisera as performed in our previous publication to resolve these two cases.8 In case 1, the patient's plasma presented weak reactivity (2+) with A RBCs and strong reactivity (4+) with B RBCs in the reverse phenotyping. This result was concordant with the forward phenotyping but attracted our attention since a strong reactivity (4+) of the O blood type plasma is expected with A1 and B RBCs in the reverse phenotyping. We used a modified protocol in which the patient RBCs were tested with a HTMA anti-A (2048) and anti-A,B (1024) in column agglutination technology (CAT). We observed a weak reaction (+1) with anti-A and a strong (+4) with anti-A,B. These data are concordant with the resultsof the eluate testing by the Lui freeze-thaw elution technique.5
Extended investigations performed in tubes reveal the presence of a cold autoantibody anti-I reacting with adult A and O RBCs but not with cord blood O RBCs. This cold anti-I antibody created a false concordance between the forward and the reverse phenotyping leading to a misinterpretation of the patient ABO type as O. This false concordance has hidden the absence of the A antigen in the patient RBCs. This fact agrees with our previous observations in which we show an anti-A1 irregular antibody with the same behavior.8
In case 2, the patient plasma did not react with A1 RBC but showed strong reactivity (4+) with B RBC in the reverse phenotyping in CAT, with no discrepancies in the forward and reverse phenotyping. In a third and fourth blood sample, the patient RBCs showed a weak reaction with anti-A (2+ and 1+, respectively) in CAT. The fifth and sixth samples showed the complete disappearance of the A antigen from the RBCs. This result attracted our attention to investigate the potential disappearance of the A antigen. We used a modified protocol using high titers of anti-A and anti-A,B antisera in CAT.8 We observed a strong reaction of the patient RBC with a titer equal to or higher than 2048 and 1024, for anti-A and anti-A,B, respectively. These data are concordant with the results of the eluate testing. Extended investigations performed in tubes reveal a cold autoantibody anti-IH showing a weak reaction with A RBC but a strong reaction with adult O RBC but not with cord blood O RBC. This cold anti-IH antibody did not interfere in the forward and reverse phenotyping. However, we observed the disappearance of the A antigen in the patient RBC.
The methods used routinely failed in detecting the A antigen in part of the samples analyzed. However, detecting the A antigen in those samples in which the routine methods failed was achieved by antisera anti-A and anti-A,B with high titers (Table 2). The data reported here demonstrate that the temporary loss of the A antigen in patients with myeloid malignancies is not total, and antisera might detect it in high titers in the different phases of this disease. Therefore, we can infer that HTMA ABO are helpful to resolve discrepancies between forward and reverse phenotyping resulting from weak expression or even loss of antigens from the RBCs.
There are different explanations to justify the loss of ABO antigens from RBC among patients suffering from myeloid malignancies. One tentative explanation for the loss of A and B antigens in oncohematological diseases is the 9;22 chromosomal translocation, but this phenomenon is uncommon in AML.9,10 Other researchers believe that the absence of the A antigen is consequent to H antigen loss, but our data do not agree with this proposition.2
Our data show that patients with the apparent loss of A antigen preserved the expression of the H antigen in the RBC membrane, which was detected by the strong reactivity with anti-H lectin. This observation was consistent with the weak expression of the A antigen undetected by routine tests but detected in both cases by the HTMA anti-A and anti-A,B. In conclusion, the data of these reports demonstrate that HTMA can detect the weak expression of ABO antigens in cases with transitory weak antigen expression in patients with myeloid malignancies.
FundingThis work was supported by FAPESP São Paulo Research Foundation(#2009/17540-2 and 2012/07716-9 to LCM).
Funding disclosureThe opinions, assumptions, and conclusions or recommendations expressed in this material are the authors' responsibility and do not necessarily reflect the views of the FAPESP. The funders had no role in study design, data collection, and analysis, decision to publish, or manuscript preparation.