Despite advances in health care for sickle cell disease patients, as well as in the improvement in reproductive issues mainly in women with the disease, pregnancy is still a challenge, both for the mother and the child, with high rates of maternal and fetal morbidity and mortality. Besides their chronic hemolytic status and vaso-occlusive events that confer systemic complications, pregnant women also have higher rates of pain episodes, infections, abortion, intrauterine growth retardation, pre-term births, eclampsia, stillbirth and the hemolysis, elevated liver enzymes and low platelets syndrome. The physiologic mechanisms of the disease in pregnancy are still unknown and chronic inflammatory responses may interfere in the adverse outcomes. The cytokine and chemokine profiles in pregnancy with sickle cell disease remain unknown. The aim of this study was to evaluate the cytokine profile of the inflammatory response of pregnant women with sickle cell disease.
MethodBlood samples from 20 pregnant women with sickle cell disease, 24 women with sickle cell disease in steady state, 16 healthy pregnant women and a control group with 9 women at childbearing age were assayed for interleukin-6.
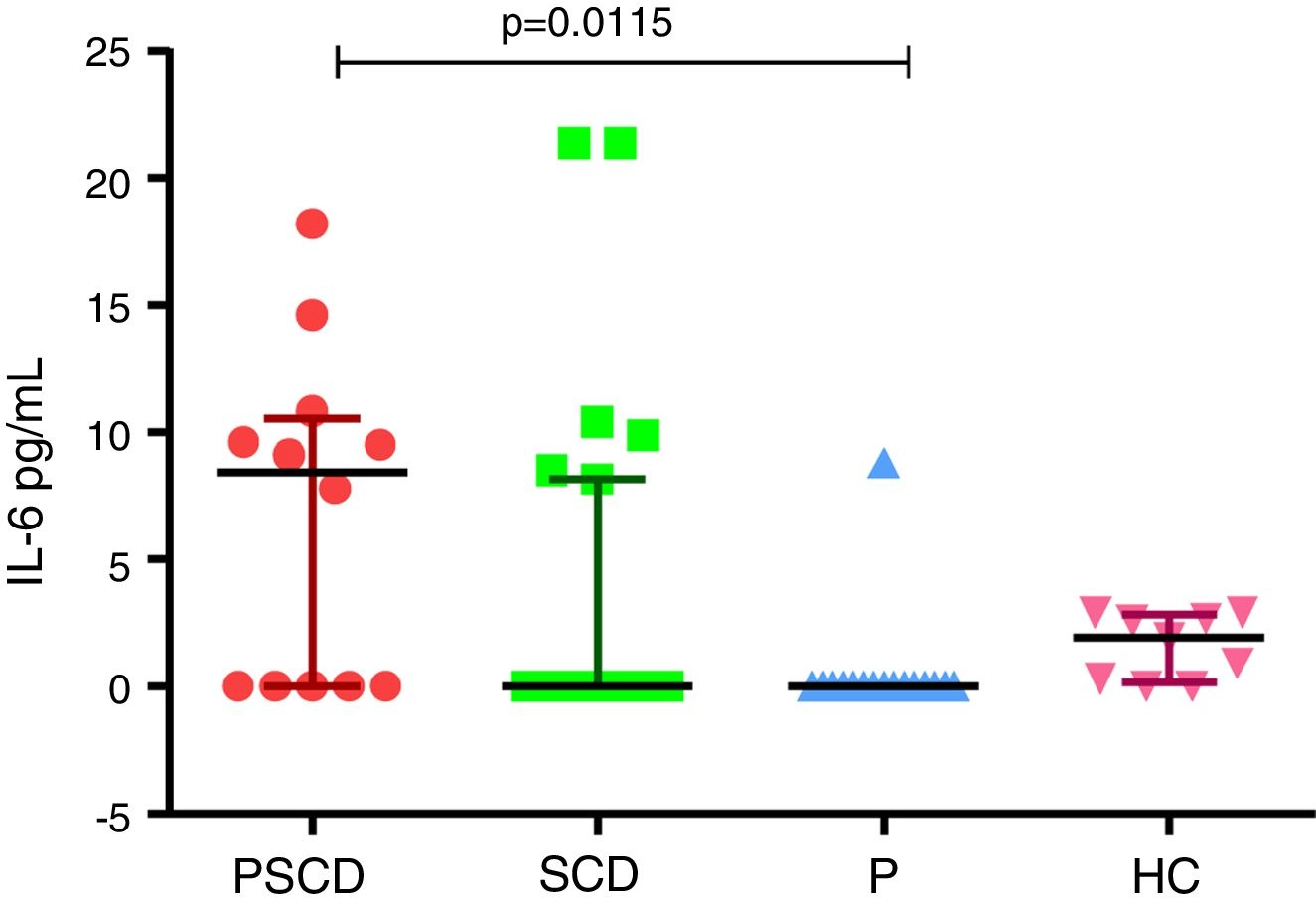
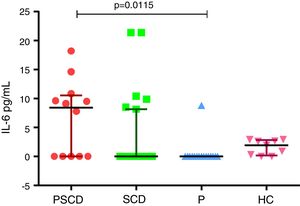
Main resultsPregnant women with sickle cell disease presented high serum levels of interleukin-6, compared to healthy pregnant women (p=0.0115).
ConclusionThese data suggest that the increased production of interleukin-6 may occur during pregnancy with sickle cell disease and that the role of this cytokine in the sickle cell disease pathophysiology and pregnancy complications should be further studied.
Sickle cell disease (SCD), one of the most common hemoglobinopathies, causes an alteration in the normal activities of different systems, characterized by a chronic inflammatory state, with hemolytic anemia and vaso-occlusion, in different organs and tissues.1
Epidemiological studies demonstrate that pregnant women with SCD have increased mortality and obstetric risk. These women also present a worse clinical course with hemolysis, higher risk of infections and recurrent vaso-occlusive crisis (VOC), with chronic organ damage.2
Although individuals with SCD have elevated baseline inflammation and endothelial activation, the acute phase response has not been evaluated among pregnant women in this condition. Markers of endothelial dysfunction and inflammation, such as the pro-inflammatory cytokines IL6, may be elevated during complications in pregnancy. The physiopathology pathways of the disease are multifactorial and the participation of cytokines is reported in many studies in different situations, but the reports during pregnancy are still scarce.3,4
The IL-6 cytokine, corresponding to the acute phase of inflammation, is at high levels in patients with SCD, both in acute events and during steady state situations, probably secondary to subclinical and persistent SCD inflammation.5 There are studies on non-complicated pregnancies without SCD, with the elevation of markers of acute inflammatory activity, such as cytokines.6–8
Therefore, there are no studies related to the possibility of investigating the participation of IL-6 as a marker of complication in the pregnancy of women with SCD, since gestation is a period in which there is a higher morbidity and mortality in women with SCD.9–12
The importance of studying cytokines in pregnancy has been established, but in SCD the panel of cytokines in pregnancy has still not been defined.
In this study, we aimed to describe inflammatory cytokine IL-6 in pregnant women with SCD and to compare them with control groups.
MethodsThis exploratory study was conducted at the Instituto de Medicina Integral Prof. Fernando Figueira (IMIP), in Recife in northeastern Brazil, from September 2014 to October 2015. The IMIP is a tertiary hospital with expertise in high-risk pregnancy and assistance to pregnant women with SCD.
This study evaluated the serum levels of IL-6 in four groups: 20 pregnant women with SCD, 24 women with SCD in steady-state condition, 16 healthy pregnant women without SCD and 9 healthy non-pregnant women without SCD.
The serum levels of IL-6 analyses were performed during the three gestational trimesters and the second trimester was chosen because it represented the lowest loss in the follow-up. The data were collected from a questionnaire and the women had similar age, family income, schooling, age of menarche and parity, making them a comparable group.
The women with SCD were all diagnosed with hemoglobin S (Hb SS) by high-performance liquid chromatography (HPLC). None of the participants had reported recent infection and all the women with SCD were only taking folic acid.
The blood samples of non-pregnant women with SCD were collected during the steady state, whereas the blood samples from pregnant women with SCD were collected at least 30 days after any blood transfusion. This time interval was determined to assure there was no production and liberation of IL-6 secondary to an inflammatory response from the mechanical process of transfusion, with consequent changes in the baseline values of IL-6.
The blood samples from groups of the healthy pregnant women without SCD and of the healthy non-pregnant women were from a sample database of women with similar ages and socioeconomic status from the Translational Research Laboratory at IMIP.
Blood samples were drawn in ethylenediaminetetraacetic acid (EDTA) tubes at study inclusion and were kept on ice until being processed within 30minutes of collection. Plasma was obtained by centrifugation for 10minutes at 1000 x g and stored at ≤−80°C for subsequent quantification of cytokines.
Serum levels of cytokine were determined using the enzyme linked immunosorbent assay (ELISA), using the BD OptEIA™ Human ELISA Set (BD Biosciences, San Diego, CA, USA), according to the manufacturer's instructions. Immediately after performing the assays, the absorbances of the reaction were read in the plate reader at the 450-nm wavelength (Human®, Germany). The concentration of cytokine in the samples was calculated from standard curves using the absorbance values of the standards provided by the manufacturer. Concentrations were expressed in pg/mL.
Cytokine levels were not normally distributed according to the D’Agostino-Pearson omnibus normality test, and the Kruskal–Wallis test was used to compare multiple groups. Statistical significance was defined as p<0.05. Data analysis was performed using the GraphPad Prism v6.0 (GraphPad Software, San Diego, CA, USA) (Figure 1).
IL-6 levels in pregnancy with sickle cell disease and controls. Samples with non-detectable cytokine level were considered to be zero pg/mL. PSCD: pregnancy with sickle cell disease; SCD: sickle cell disease women in steady state; P: healthy pregnant women; HC: healthy control women.
The study was approved by the IMIP Research Ethics Committee (CAAE: 28045814.1.00005201) and written informed consent was obtained from all participants.
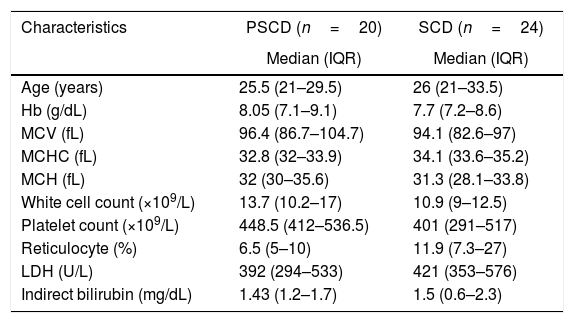
ResultsThe hematological parameters of women with SCD had similar aspects, as shown in Table 1. None of the participants had reports of recent infection. All women with SCD were taking folic acid and all pregnant women with SCD were transfused during pregnancy. The women with sickle cell disease were not under use of hydroxyurea and were in steady state.
Hematological parameters of the SCD population.
| Characteristics | PSCD (n=20) | SCD (n=24) |
|---|---|---|
| Median (IQR) | Median (IQR) | |
| Age (years) | 25.5 (21–29.5) | 26 (21–33.5) |
| Hb (g/dL) | 8.05 (7.1–9.1) | 7.7 (7.2–8.6) |
| MCV (fL) | 96.4 (86.7–104.7) | 94.1 (82.6–97) |
| MCHC (fL) | 32.8 (32–33.9) | 34.1 (33.6–35.2) |
| MCH (fL) | 32 (30–35.6) | 31.3 (28.1–33.8) |
| White cell count (×109/L) | 13.7 (10.2–17) | 10.9 (9–12.5) |
| Platelet count (×109/L) | 448.5 (412–536.5) | 401 (291–517) |
| Reticulocyte (%) | 6.5 (5–10) | 11.9 (7.3–27) |
| LDH (U/L) | 392 (294–533) | 421 (353–576) |
| Indirect bilirubin (mg/dL) | 1.43 (1.2–1.7) | 1.5 (0.6–2.3) |
SCD: sickle cell disease; PSCD: pregnant with SCD; IQR: interquartile range (25–75th); Hb: hemoglobin; MCV: mean corpuscular volume; MCHC: mean corpuscular hemoglobin concentration; MCH: mean cell hemoglobin; LDH: lactate dehydrogenase.
In our study, the analysis of IL-6 had higher levels in pregnant women with SCD, compared to pregnant women without SCD (p=0.0115). There were no statistical differences between the other groups of comparison. Graph 1 summarizes the data on IL-6 in the different groups.
DiscussionSickle cell disease, globally the most common genetic disorder, is a chronic inflammatory condition characterized by elevated levels of inflammatory cytokines, IL-6 being a cytokine associated with pro-inflammatory effects also on tissue homeostasis, regeneration, and metabolism in different diseases.13–15
Pregnancy is also a situation where a perfect regulation of the maternal immune system results in successful outcomes for the mother and fetus. In some pathological conditions such pre-eclampsia, rheumatologic diseases and preterm, immune responses are altered.16–19
The physiological changes of pregnancy will exacerbate the pathophysiological state in SCD. Pregnancy worsens anemia, increases risk of infection, exacerbates the procoagulant tendency of pregnancy and increases the frequency of pain crises. There are some potential mechanisms associated with this, but the exact mechanism is still unknown.20–23
The current clinical management of pregnant women with sickle-cell anemia is still a challenge, as these women could be at risk of becoming critically ill in this period. There is an increased risk of a longer hospitalization stay, an elevated risk of deaths and worse outcomes for the women and for the concepts, such as preterm, abortions, gynecological bleeding and small size for the gestational age.11,24–26
Despite the importance of the clinical aspects in pregnant women with SCD, immunologic biomarkers may be implicated in the unfavorable outcomes in this group of patients that have not yet been elucidated. The results presented here can be attributed to the inflammatory activity of SCD overlapping the intrinsic inflammation from gestation.17,27
Early identification of biomarkers in pregnant women with SCD who will present gestational complications is critical and may be useful in predicting which of them will present unfavorable outcomes. Currently, there is no evidence of biomarkers in pregnancy in patients with SCD. The IL-6 is a cytokine involved in the regulation of the acute phase inflammatory responses, which, in addition to vaso-occlusive events, are higher in SCD individuals, when compared to healthy ones.28
In the context of pregnancy, IL-6 is required for the immune adaptations in healthy pregnancies and there are reports on elevated levels of IL-6 at the onset of spontaneous abortion, preterm delivery, chorioamnionitis and other obstetric complications.29
Although this study is an exploratory one, presenting a limited number of participants, it was possible to verify a statistical difference in serum levels of IL-6 between pregnant women with SCD and pregnant women without SCD, making it possible to assume that there may exist some relationship between the unfavorable outcomes in pregnant women and SCD, when compared to healthy ones.
The increase in the inflammatory response verified in pregnant women with sickle cell disease could be attributed to the SCD itself, as it is a disease with continuous and chronic inflammatory stimuli in patients afflicted by it. The inflammatory aspects of SCD have already been described in previous studies, but on pregnancy there is still a lack of articles.30–35
Distinguishing the precise role of IL-6 in a SCD pregnancy is a diagnostic challenge in these critically ill patients. This determination is important, with distinct differences in the management and prognosis of each specific clinical entity. Biomarkers have increasingly been utilized in clinical diagnosis and decision making.36,37
Due to the small size of the sample analyzed here, and consequent impossibility to infer causality, the results suggest that both pregnancy and SCD may play a role in the elevation of this cytokine and this study is one of the first ones to describe this field of investigation.
ConclusionBoth pregnancy and SCD may have played a role in the IL-6 elevation. It is also important to know whether these cytokines are consistently elevated and there is a need to establish a panel of validated biomarkers in pregnant women with SCD. More studies with large samples are necessary to verify the prognostic and predictive value of biomarkers in pregnant women with SCD.
Financial supportNone declared.
Conflicts of interestThe authors declare no conflicts of interest.