Sickle cell anemia is a chronic inflammatory disease characterized by an increased production of proinflammatory cytokines including tumor necrosis factor-alpha. Hydroxyurea, by decreasing the polymerization of hemoglobin, reduces inflammatory states. The effect of the genetic polymorphisms of sickle cell patients on tumor necrosis factor-alpha levels remains unknown.
ObjectiveThe aim of this study was to investigate the association of tumor necrosis factor-alpha levels with β-globin haplotypes and the use of hydroxyurea.
MethodsA cross-sectional study was performed of 67 patients with sickle cell anemia diagnosed at steady-state in a referral hospital in Fortaleza, Ceará, Brazil. A group of 26 healthy individuals was used as control. βS haplotype analysis was performed by restriction fragment length polymorphism-polymerase chain reaction. The tumor necrosis factor- alpha levels were measured by the enzyme-linked immunosorbent assay test. Laboratory data (complete blood count and fetal hemoglobin) and information regarding the use of hydroxyurea were obtained from medical records. Statistical analysis was performed using R software with the Kruskal-Wallis and Mann–Whitney tests. Statistical significance was established for p-values<0.05 for all analyses.
ResultsThe mean age of the participants was 35.48years. Patients with sickle cell anemia had significantly higher tumor necrosis factor-alpha levels than controls (p-values<0.0001). Tumor necrosis factor-alpha levels were lower in sickle cell anemia patients who were receiving hydroxyurea treatment than those who were not (p-value=0.1249). Sickle cell anemia patients with Bantu/n genotype had significantly higher levels than patients with the Bantu/Benin genotype (p-value=0.0021).
ConclusionIn summary, βS-globin haplotypes, but not hydroxyurea therapy, have a role in modulating tumor necrosis factor-alpha levels in sickle cell anemia adults at steady-state. Many previous studies have investigated prognosis and inflammatory states in sickle cell anemia patients, but the discovery that tumor necrosis factor-alpha levels vary according to the genetic polymorphism of the patient is a new finding. © 2014 Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular. All rights reserved.
Sickle cell anemia (SCA) is a genetic disorder characterized by a point mutation in the beta globin gene generating, when homozygous, an abnormal hemoglobin known as hemoglobin S (Hb S).1,2 Clinical manifestations result from the tendency of Hb S to polymerize in the deoxygenated state, causing vascular obstructions and ischemia, and consequent painful crises associated with episodes of chronic hemolysis.1,3
The vaso-occlusive crisis is the most common symptom in SCA patients, manifesting in 50% of cases.4 This event is mainly attributed to abnormal adhesion of sickled red blood cells, granulocytes and platelets to the vascular endothelium, with production of proinflammatory mediators that stimulate the production of endothelial adhesion molecules.5 SCA is considered a chronic inflammatory disease characterized by an increased production of proinflammatory cytokines such as interleukin (IL)-6, IL-8 and tumor necrosis factor-alpha (TNF-α).6,7
TNF-α is a cytokine with a large variety of properties that is involved in the activation of endothelial cells and leukocytes, the stimulation of macrophages and the recruitment and chemotaxis of inflammatory cells;8 it has been implicated in the pathogenesis of various acute and chronic states, including sepsis, chronic infections and inflammatory conditions. Additionally it plays an important role in the synthesis of acute phase proteins and in the expression of adhesion molecules by the vascular endothelium.9 In SCA, TNF-α has been linked as a risk factor for the occurrence of painful crises, as well as participating in the occlusion of the microcirculation.10
Although the disease has a genetic cause common to all carriers, patients can manifest a variable clinical picture including severe alterations in the function of vital organs.5,9 Several factors may modulate the clinical features of SCA, such as fetal hemoglobin (Hb F), the β-globin haplotypes and co-association with thalassemia.11 The different haplotypes may influence Hb F levels and the clinical presentation of SCA patients. The Senegal haplotype, by producing the highest levels of fetal hemoglobin in the blood (>15%), is associated with a less severe clinical evolution of SCA and a lower occurrence of organic damage. The clinical picture of the Benin haplotype is of intermediate severity, with Hb F levels between 5% and 15%. The Bantu or CAR haplotype is associated with greater clinical severity and the lowest Hb F levels (<5%).12,13
Hydroxyurea (HU), used in the treatment of SCA, is a chemotherapeutic agent that increases the concentration of Hb F, thereby decreasing the polymerization of hemoglobin in deoxygenated states and reducing sickling.14 HU contributes to reduce the plasmatic expression of adhesion molecules as well as clinical episodes of acute chest syndrome and painful crises.15 Despite the numerous benefits that the use of HU can bring to users, not all patients have sufficient clinical and laboratory criteria to be included in the treatment protocol.16 In addition, some patients refuse treatment because of the potential risks and side effects related to the use of HU.
In this context, the present study aimed to evaluate the profile of the proinflammatory cytokine TNF-α and its association with the haplotypes of the beta globin S and the use of HU.
MethodsA cross-sectional study was carried out with 67 volunteer patients of both genders, aged from 18-66 years with clinical and laboratory diagnosis of SCA, attended in the hematology service of a hospital in Fortaleza, Ceará, Brazil. Patients were at steady-state in accordance to criteria established by Ballas17 and were stratified in groups according to genotype and whether they received HU or not: Bantu¿n [patients with Bantu¿Bantu (n=31) and Bantu¿Atypical (n=6) genotypes]; Bantu¿Benin (n=22) and Benin¿Benin (n=8). A group of 26 healthy individuals was used as a control group.
The analysis of the haplotype cluster βS was performed by restriction fragment length polymorphism-polymerase chain reaction (PCR-RFLP). The level of the cytokine TNF-α was measured by the enzyme-linked immunosorbent assay (ELISA) test using cytokine kits (BD Biosciences ®). Laboratory data (complete blood count and Hb F concentration) and information regarding the use of HU were obtained from medical records.
The study was approved by the Research Ethics Committee of Hospital Universitário Walter Cantídio (HUWC) and was conducted in accordance with the Declaration of Helsinki as revised in 2008. Informed consent forms were signed by all participants.
Statistical analysis was performed with R software using the Kruskal-Wallis and Mann–Whitney tests. The results are expressed as means±standard deviation (SD). The level of significance was set at 5% (p-value<0.05).
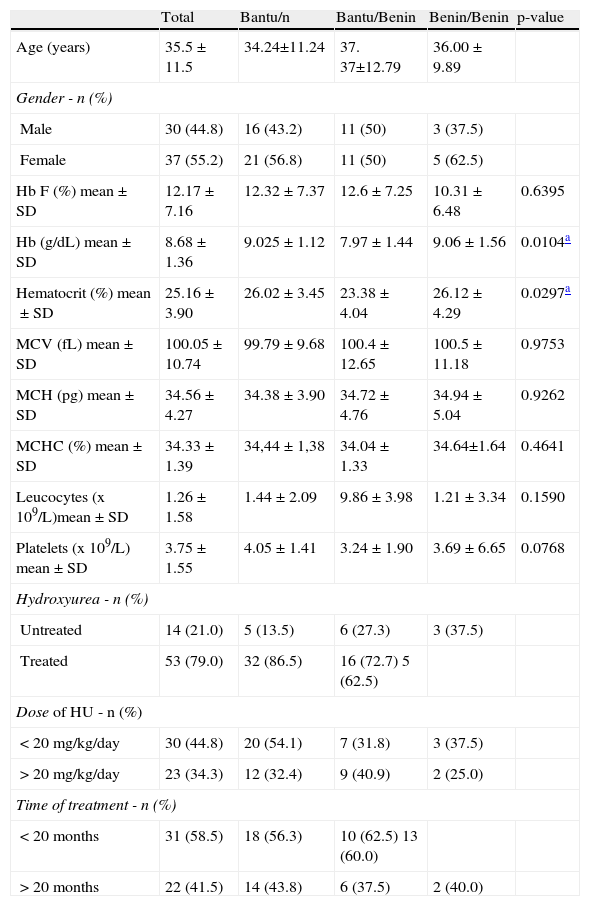
ResultsThe clinical details of the steady-state SCA patients according to genotypes and the use of HU are shown in Tables 1 and 2, respectively. Of the 67 patients included in the study, 55.2% were female and 44.8% were male. The mean age was 35.48 (±11.55) years. The patient group had a mean Hb concentration of 8.68g/dL (±1.36g/dL), hematocrit (Ht) 25.16% (±3.9%) mean corpuscular volume (MCV) 100.05fL (±10.74fL), mean corpuscular hemoglobin (MCH) 34.56pg (±4.27pg), mean corpuscular hemoglobin concentration (MCHC) 34.33% (±1.39%), leukocytes 1.26×109/L (±1.58×109/L), platelets 374.90×109/L (±154.59×109/L), Hb F 12.17% (±7.16%) and Hb S 81.32% (±7.99%). The Bantu/n genotype was the most common; it was expressed in 37 of the patients (55.2%), followed by Bantu/Benin with 22 (32.8%) and Benin/ Benin with eight patients (11.9%). A total of 53 (79%) patients were treated with HU: 30 (44.8%) recieved a dose less than 20mg/kg/day and 23 (34.3%) recieved a larger dose; 31 (58.5%) received HU for a period of less than 20 months and 22 (41.5%) for a longer period (Table 1).
Clinical details of steady–state sickle cell anemia patients (n=67) according to genotype.
| Total | Bantu/n | Bantu/Benin | Benin/Benin | p-value | |
| Age (years) | 35.5±11.5 | 34.24±11.24 | 37. 37±12.79 | 36.00±9.89 | |
| Gender - n (%) | |||||
| Male | 30 (44.8) | 16 (43.2) | 11 (50) | 3 (37.5) | |
| Female | 37 (55.2) | 21 (56.8) | 11 (50) | 5 (62.5) | |
| Hb F (%) mean±SD | 12.17±7.16 | 12.32±7.37 | 12.6±7.25 | 10.31±6.48 | 0.6395 |
| Hb (g/dL) mean±SD | 8.68±1.36 | 9.025±1.12 | 7.97±1.44 | 9.06±1.56 | 0.0104a |
| Hematocrit (%) mean±SD | 25.16±3.90 | 26.02±3.45 | 23.38±4.04 | 26.12±4.29 | 0.0297a |
| MCV (fL) mean±SD | 100.05±10.74 | 99.79±9.68 | 100.4±12.65 | 100.5±11.18 | 0.9753 |
| MCH (pg) mean±SD | 34.56±4.27 | 34.38±3.90 | 34.72±4.76 | 34.94±5.04 | 0.9262 |
| MCHC (%) mean±SD | 34.33±1.39 | 34,44±1,38 | 34.04±1.33 | 34.64±1.64 | 0.4641 |
| Leucocytes (x 109/L)mean±SD | 1.26±1.58 | 1.44±2.09 | 9.86±3.98 | 1.21±3.34 | 0.1590 |
| Platelets (x 109/L) mean±SD | 3.75±1.55 | 4.05±1.41 | 3.24±1.90 | 3.69±6.65 | 0.0768 |
| Hydroxyurea - n (%) | |||||
| Untreated | 14 (21.0) | 5 (13.5) | 6 (27.3) | 3 (37.5) | |
| Treated | 53 (79.0) | 32 (86.5) | 16 (72.7) 5 (62.5) | ||
| Dose of HU - n (%) | |||||
| < 20mg/kg/day | 30 (44.8) | 20 (54.1) | 7 (31.8) | 3 (37.5) | |
| > 20mg/kg/day | 23 (34.3) | 12 (32.4) | 9 (40.9) | 2 (25.0) | |
| Time of treatment - n (%) | |||||
| < 20 months | 31 (58.5) | 18 (56.3) | 10 (62.5) 13 (60.0) | ||
| > 20 months | 22 (41.5) | 14 (43.8) | 6 (37.5) | 2 (40.0) | |
MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration.
Statistical analysis using Kruskal-Wallis test
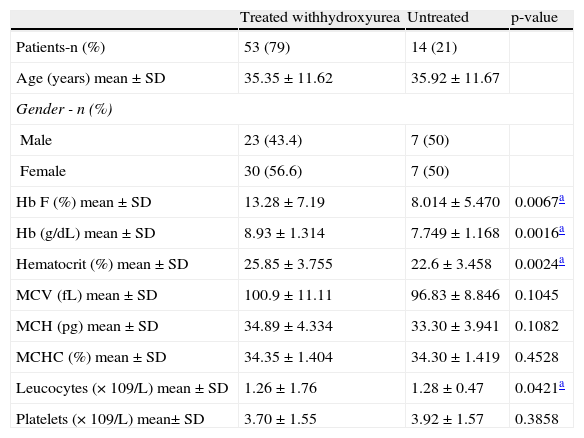
Clinical details of steady-state sickle cell anemia patients (n=67) according to use of hydroxyurea.
| Treated withhydroxyurea | Untreated | p-value | |
| Patients-n (%) | 53 (79) | 14 (21) | |
| Age (years) mean±SD | 35.35±11.62 | 35.92±11.67 | |
| Gender - n (%) | |||
| Male | 23 (43.4) | 7 (50) | |
| Female | 30 (56.6) | 7 (50) | |
| Hb F (%) mean±SD | 13.28±7.19 | 8.014±5.470 | 0.0067a |
| Hb (g/dL) mean±SD | 8.93±1.314 | 7.749±1.168 | 0.0016a |
| Hematocrit (%) mean±SD | 25.85±3.755 | 22.6±3.458 | 0.0024a |
| MCV (fL) mean±SD | 100.9±11.11 | 96.83±8.846 | 0.1045 |
| MCH (pg) mean±SD | 34.89±4.334 | 33.30±3.941 | 0.1082 |
| MCHC (%) mean±SD | 34.35±1.404 | 34.30±1.419 | 0.4528 |
| Leucocytes (×109/L) mean±SD | 1.26±1.76 | 1.28±0.47 | 0.0421a |
| Platelets (×109/L) mean± SD | 3.70±1.55 | 3.92±1.57 | 0.3858 |
MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration.
Statistical analysis using Kruskal-Wallis test.
Serum levels of TNF-α were significantly higher in SCA patients compared to healthy individuals (p-value<0.0001; Figure 1).
Regarding the use of HU, TNF-α levels were decreased in patients who were receiving treatment compared to those who were not (p-value=0.1249; Figure 2). Patients who were receiving HU at a dose of<20mg/kg/day did not have significantly different TNF-a levels compared to those who received doses>20mg/kg/day (p-value=0.3045). Regarding the duration of treatment with HU, patients receiving the drug for a period greater than or equal to 20 months showed no statistical difference in the TNF-α levels when compared to patients who were treated with HU for less than 20 months (p-value=0.3034).
When comparing TNF-α levels between βS-globin haplotypes, it was observed that the Bantu/n group had significantly higher TNF-α levels than the Bantu/Benin group (p-value=0.0021; Figure 3).
DiscussionThe recurrent vaso-occlusion-ischemic processes induce a chronic inflammatory response in SCA patients, which is characterized by high levels of proinflammatory cytokines that activate the endothelium.18 The activated endothelium plays an important role in the initiation and propagation of the chronic inflammatory process that produces cytokines, including IL-8, IL-1 and IL-6. They contribute to the pancellular activation, which results in high circulating levels of the numerous inflammatory molecules found in SCA patients. Thus, there is a vicious cycle between production of inflammatory mediators and cell adhesion to the endothelium, leading to a chronic inflammation process.19 The results of the present study show that TNF-α levels were more elevated in patients with SCA compared to healthy individuals. Similar results were found by other studies in SCA patients at steady-state.20,21 This cytokine is fundamental for inflammatory diseases because it up-regulates the cytokine cascade responsible for inflammation.
Regarding HU therapy, patients receiving treatment had lower TNF-α levels compared to patients who were not, but this difference was not significant. These results confirm those reported by Tavakkoli et al.19 who found no difference in the TNF-α serum levels in SCA patients at steady-state in relation to receiving HU or not. Furthermore, the authors found no difference between the vaso-occlusive crises of these patients. On the other hand, Lanaro at al.,20 in a study with 50 SCA adult patients at steady-state on HU therapy, showed that they had significantly lower TNF-α levels compared to 26 untreated patients. So, the effect of HU therapy on the release of inflammatory mediators is not well understood.
In the current study, a higher prevalence of the Bantu haplotype was found compared to the Benin haplotype. This result is in agreement with the study by Silva et al.12 who used the same population as ours and explained this result by the origins of the black population brought to the state of Ceará.
Studies of several different ethnic groups of SCA patients with distinct hematological characteristics suggested that the βS-globin gene cluster haplotype may be useful as a predictor of disease severity.22–25 Furthermore,Bakanay et al.26 demonstrated that the βS-globin gene cluster haplotype, independent of the Hb F level, is correlated to survival of SCA patients on HU therapy. In the present study, a significant increase of TNF-α levels was observed in the Bantu/n group compared to the Bantu/Benin group. This result can be explained by the fact that patients with the Bantu haplotype have a tendency of having more severe clinical manifestations because of the increased concentration of Hb S and decreased concentration of Hb F.27 Sickled red blood cells exhibit abnormal adhesion to the vascular endothelium, thus contributing to the expression of adhesion molecules and amplification of inflammation by proinflammatory cytokines such as TNF-alpha.8,28
ConclusionSCA expresses factors that influence the clinical features of the patients. In this study one of these factors was evidenced, showing the modulatory role of the β-globin haplotypes on TNF-α levels in SCA adults at steady-state. Many previous studies have investigated the prognosis and inflammatory state of SCA patients, but the discovery that TNF-α levels vary according to the genetic polymorphism of the patient is a new finding.
Conflicts of interestThe authors declare no conflicts interest.