Autoimmune haemolytic anaemia (AIHA) is an autoimmune disorder that can present in primary or secondary forms. The literature looking at impact of baseline fluorescent antinuclear antibody (FANA) positivity on outcomes of AIHA patients is infrequent.
ObjectiveTo study the impact of baseline FANA positivity in patients with primary AIHA.
MethodA prospective cohort study involving 29 consecutive primary AIHA patients presenting to the Haematology department from 2013 to 2015 was analysed. After recording baseline investigations including fluorescent ANA, all patients were treated as per the standard therapeutic protocols. Clinical remission, disease free survival, relapse, mortality were compared between the FANA positive and FANA Negative AIHA groups.
ResultsBaseline FANA positivity was found in 17 patients (58.62%). Both the groups were comparable in terms of age, sex, Hemoglobin, LDH at presentation, number of lines of treatment needed and duration of follow up. Evan's syndrome was seen in six of FANA positive patients which was statistically significant (0 v/s 6, p = 0.023). FANA positive patients had significantly higher rates of relapse per patient month follow up (1.22 v/s 3.57, p = 0.023) and lower rates of complete response (83.33% v/s 35.29%, p = 0.0118) and relapse free survival at five years. Morbidity and mortality were numerically higher in FANA positive patients.
ConclusionBaseline FANA positivity among AIHA patients was found to be associated with lower complete response rates and higher relapse rates with possible higher rates of morbidity. Presence of FANA will give us prognostic value and help us in deciding the treatment options.
Autoimmune hemolytic anemia (AIHA) is an acquired heterogeneous autoimmune disorder characterized by the development of antibodies directed against antigens on autologous erythrocytes. It is a relatively rare disorder, with an estimated incidence of 1–3 cases in 100,000 persons per year in the west.1 Exact incidence in India is not known.2 Most common are hemolytic anemias mediated by autoantibodies that bind to red cells at 37̊° C, which accounts for 65–70% of AIHAs, also called as Warm Antibody AIHA or WAIHA.43 Most of the patients have the IgG type of autoantibody.4 Autoimmune hemolytic anaemia can occur as a primary (idiopathic) disorder or occur in association with other autoimmune or other disorders (secondary AIHA). Secondary forms of AIHA may account for 40% to 50% of all the patients with AIHA. The diagnosis of AIHA may precede the recognition of another underlying disorder, commonly Systemic Lupus Erythematosus (SLE) or chronic lymphoproliferative diseases (CLPD).1,4,5 Up to 7% of patients may have co-existing autoimmune thrombocytopenia or neutropenia, a condition also termed as Evan's syndrome.1,5,6
Once a diagnosis of AIHA is established, a detailed laboratory assessment has to be made to look for co-existing primary diseases, such as autoimmune diseases or chronic lympho-proliferative diseases. One of the investigations routinely performed is the detection of the antinuclear antibody by the immunofluorescence technique (FANA). If the FANA test is positive, further testing for Anti-ds DNA and ANA profiling can be done. Many times patients are positive for FANA with no obvious evidence of underlying connective tissue disease. In such cases, it is not very clear whether the FANA plays a role in the pathogenesis of the disease. There are reports that suggest these patients may in the future progress to develop SLE or Anti-Phospholipid Antibody Syndrome (APS) and may have more complications.7 We wanted to see if the nature of the disease differs in patients with FANA Positive AIHA from that of FANA Negative AIHA, in terms of disease presentation, treatment response, relapse rates and complications.
MethodThe study was performed at the Department of Adult Hematology at a tertiary care teaching hospital. It is a prospective cohort study involving 29 primary AIHA patients among the 32 consecutive AIHA patients who presented to the department from 2013 to 2015. After the preliminary analysis, the patients were followed up for 5 years (until 2020). The serological associations with the FANA, treatment administered and the outcomes were progressively reviewed in all cases. The patients were evaluated with the complete blood count (CBC), lactate dehydrogenase (LDH), liver and renal functions, along with direct antiglobulin test (DAT) and indirect antiglobulin test (IAT) using polyspecific reagents (Matrix Gel system, Tulip diagnostics (P) Ltd., India at baseline. Further immunohematological classifications could not be made due to the lack of monospecific reagents in our lab. All patients with atypical cells in the peripheral smear underwent bone marrow studies. Patients with lymphadenopathy were subjected to a lymph node excision biopsy with immunohistochemistry (IHC) studies. The FANA was performed in all the subjects. Patients were labeled as primary AIHA when all the above tests failed to yield an etiology. As per the departmental protocol, all the patients with mild disease were started on oral prednisolone 1 mg/kg/day, with a taper over several weeks. Those with moderate and severe disease were treated with pulse methylprednisolone therapy at 15–30 mg/kg/day for 3–5 days, followed by oral prednisolone.4,3 The steroid responses at 3 months were recorded. Those who needed persistent steroid therapy beyond 3 months of treatment initiation were treated with the addition of azathioprine or mycophenolate mofetil. Those who failed to respond, relapsed or required high-dose steroids, even at 6 to 9 months, were given Rituximab 375 mg/m2 weekly for 4 weeks. The responses were defined as follows: complete response (CR), defined as a stable hemoglobin level of > 12 g/dL, no requirement for transfusion and absence of signs of hemolysis, irrespective of the DAT test result.Partial response (PR), defined as a rise in hemoglobin levels of > 2 g/dL from the baseline and improvement of clinical and laboratory signs of hemolysis without complete biochemical resolution of hemolysis. Relapse was defined as the reappearance of AIHA activity after stopping or achieving a basic minimum maintenance therapy for at least 3 months. Sustained response was defined as the absence of any significant hemolysis off treatment or on the maintenance dose.7 The cohort was divided into FANA negative primary AIHA and FANA positive primary AIHA and parameters were compared between the two groups. The statistical analysis was made using the descriptive analysis "N-1″ Chi-squared test and unpaired t test. The study was approved by the institutional Ethics Committee through approval number IEC KMC MLR 07–14/143. Written informed consent was obtained from all the patients.
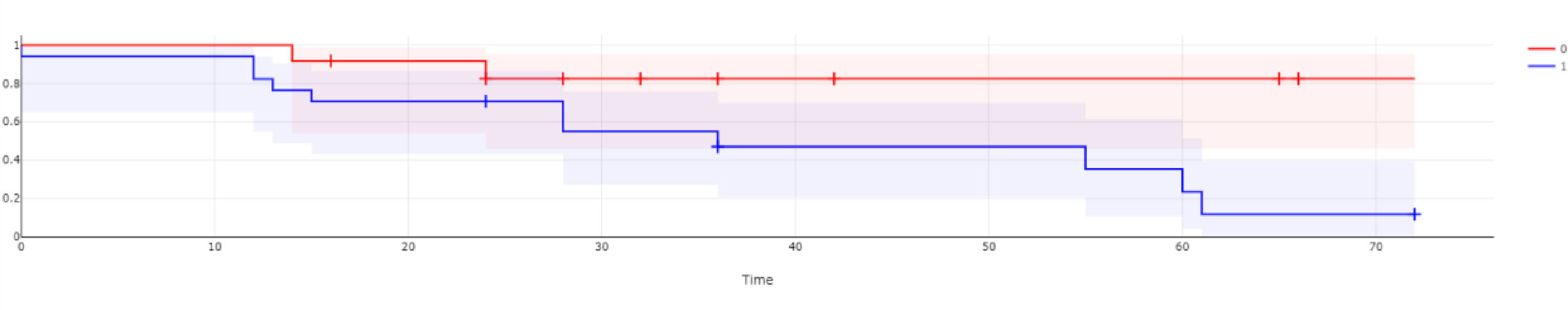
ResultsThe study involved 32 consecutive AIHA patients with DAT positive or DAT and IAT, positive by the gel technique using a polyclonal reagent, who presented to our department from 2013 to 2015. Patients with only IAT positive or both DAT and IAT negative AIHAs were excluded from the study. Autoantibody subtyping into IgG, IgM or complement-mediated AIHA was not done due to the lack of facilities. Out of the 32 patients, 29 had primary AIHA and 3 had secondary AIHA. These patients (9.6%) were excluded from the study, as they were secondary to malignancy. They were AIHA secondary to chronic lymphocytic leukemia (CLL), non-Hodgkin's lymphoma (NHL) and myeloma, respectively. The study population of 29 primary AIHA patients consisted of patients from 17 years to 78 years, with a median age of 39 years and mean of 39.9 years. The FANA positivity was observed in 58.62% (17 patients), while 41.38% (12 patients) had FANA negative primary AIHA. The age, hemoglobin percentage and LDH levels at presentation were similar in both groups (Table 1). A total of 22 patients out of 29 were females (75.89%). However, the gender distribution amongst FANA negative and FANA positive AIHA was similar (Table 2). None from the FANA negative and six from the FANA positive groups had Evan's syndrome at presentation (35.29% vs. 0%, p = 0.023, 95% CI for the difference – 5.1001% to 58.6958%), which was statistically significant. The initial steroid response rates noted at the end of 3 months of initiating the treatment were not different between the two groups (Table 2). The mean time taken to show the first response, mean number of months of follow- up and mean number of lines of treatment required to achieve sustained response were all similar between both the groups (Table 1). The proportion of complete responses with no requirement for further treatment was significantly higher in the FANA negative AIHA group at the end of the 5-year follow-up period (83.33% vs. 35.29%, p = 0.0118, with the CI at 95% for the difference 11.4408% to 69.6438%). The number of relapses per patient month follow-up was significantly lower in the FANA negative AIHA group (1.22 vs, 3.57, p = 0.0230, the CI at 95% for the difference 0.3121% to 4.3560%) (Table 2). The -free survival between the two groups was compared and presented as a Kaplan Meier survival curve in Figure 1. It shows a statistically significant (p = 0.03887) higher rate of relapse-free survival among ANA negative AIHA patients. The proportion of deaths (1 vs. 4: 8.33 vs. 23.52%, p = 0.2946 with the CI for the difference at 95% −15.2582% to 39.8894%) and the proportion of patients who developed complications including death (1 vs. 7: 8.33% vs. 41.17%, p = 0.0555, the CI at 95% −0.5465% to 56.6633%) were numerically lower in the FANA negative AIHA patient group (Table 2). Individual complications seen were Lupus nephritis, venous thromboembolic disease and hemophagocytic syndrome. (Table 2). There was no effect of gender on relapse rates in the entire group. Furthermore, the baseline hemoglobin levels did not predict the relapses in the entire cohort.
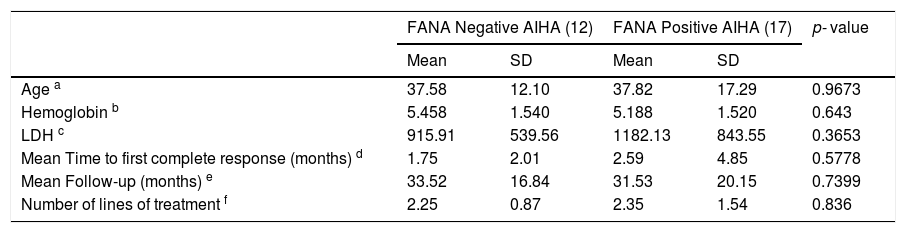
The mean baseline characteristics and follow-up details.
a, b, c – Baseline parameters.
d – Defined as normalization of hemoglobin and resolution of biochemical evidence of hemolysis.
e – Ranges 14 - 66 months in FANA negative AIHA patients and 0 - 72 months in FANA positive patients.
f – Ranges from 1 - 6 in both groups. Lines of treatment modalities used were steroids, azathioprine, rituximab, mycophenolate mofetil, cyclophosphamide and splenectomy.
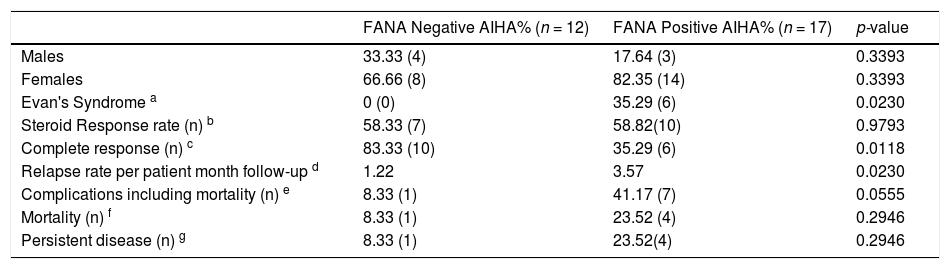
The Proportional Rate Comparison of presenting characteristics and response rates in both groups.
a- Presence of Evan's syndrome at baseline.
b- Immediate response rates to initial dose of steroids calculated at the end of 3 months of therapy.
c- Defined as number of patients who are in CR at the end of 5-year follow-up period.
d- Total number of patient month follow-up period was 407 and 560 months in FANA negative and positive groups, respectively.
e- Complications include venous thromboembolisms, lupus nephritis and hemophagocytic syndrome.
f- One FANA negative patient died 1 year after splenectomy at home and we could not elucidate the exact cause of death. Causes of death in the FANA positive patient group include sepsis and hemophagocytic syndrome.
g- Includes patients who are still requiring some form of therapy to maintain a normal hemoglobin at the end of the 5-year follow-up period. This excludes the patients who developed complications.
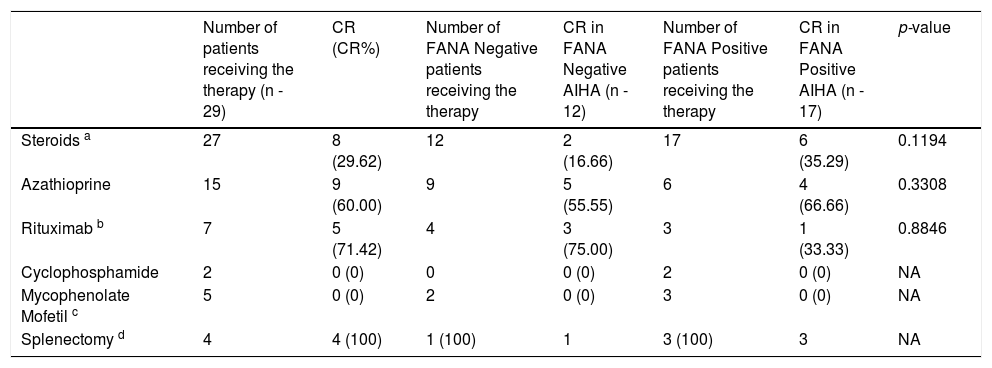
The other therapeutic modalities used were also compared, however the response rates were not statistically significant between the groups. This is possible because of the small numbers involved. The statistics are represented in Table 3. The overall steroid response rate was 66.6%. Approximately 8 patients in total achieved a CR with no relapses with only steroid therapy, translating to a 29.6% success rate. Overall Azathioprine showed an approximately 60% response rate as a second line drug. Out of 15 patients who received Azathioprine, nine patients showed a good response, with an overall response rate of 60%. Five in the FANA negative group and four in the FANA positive group achieved the CR with Azathioprine. The next frequently used drug was Rituximab. It was used in four FANA negative AIHA patients and all of them responded. One patient had a relapse 18 months after the first Rituximab course and the patient responded partially to the second course of Rituximab. Currently the patient is on Mycophenolate mofetil (MMF) maintenance therapy. Among the FANA positive AIHA, only three patients received Rituximab, with one patient achieving a CR. Two others required a splenectomy. However, the difference in the response rates between the two groups, the Rituximab response was not statistically significant. Two FANA positive AIHA patients received Cyclophosphamide with no major response. Four patients received MMF, of whom only one patient responded. Splenectomy was performed in one refractory patient in the FANA negative AIHA group. There was an immediate response, however the patient died at home after a bout of severe loose stools one year after the splenectomy and we could not evaluate the cause of death. Three FANA positive patients underwent splenectomy. All of them responded with complete remission, but one patient died post splenectomy due to extensive splanchnic and portal vein thrombosis and extensive gut necrosis.
Various Therapeutic Modalities Used and complete remission rates.
a- Steroids used were methylprednisolone, prednisolone and dexamethasone. Responses for each individual steroid is not available.
b- One patient in the FANA negative AIHA group relapsed 18 months after initial rituximab therapy and was subjected to the second course with partial response, requiring MMF maintenance therapy.
c- Used as a salvage therapy, has induced partial remission in one FANA Negative and two FANA positive patients.
d- While all the patients who underwent splenectomy achieved a CR, one FANA negative AIHA patient died one year later at home with no diagnosis and one FANA positive patient died due to extensive splanchnic and portal vein thrombosis after six months.
A study by Greidinger and Hoffman stated that about two-thirds of the patients with hemolytic anemia had hemolysis as a presenting symptom of SLE.8 The investigators could not determine how often autoimmune hemolytic anemia heralds SLE, but they found that approximately 8% of the patients with incomplete warm autoantibodies or warm hemolysins had SLE.9 Studies have shown that patients with secondary AIHA with autoimmune disorders are predisposed to renal insufficiencies and may be refractory to treatment. The prognosis is also poor in such patients.7
We wanted to evaluate the impact of the FANA positivity at the time of diagnosis in the course of the disease and the prognosis of primary AIHA patients. In one of the largest series of AIHA patients from India, Naithani et al. noted that the presence of FANA was only 12.6% (10 out of 79 subjects).10 However, in our study population, we found 58.62% FANA positivity. This is in tune with the very high incidence of autoimmune diseases in our part of the country, owing to rampant consanguineous marriages. In the same study, Naithani et al. found an increased number of females developing primary AIHA or idiopathic AIHA.10 We evaluated the association of gender and found that more than two-thirds of the total study populations were females. In our study, both the FANA positive and negative AIHA were more prevalent among females. The reason for this higher prevalence remains unknown, however most autoimmune diseases are found to be more common in females and the same was observed in our study. We found that 20 patients in the study group belonged to the age group of 25–50 years. A total of 6 patients were under 25 years, with the youngest aged 17 years, while 3 patients were over 50 years, with the eldest aged 78 years. There was no significant difference in the age distribution between the FANA positive and negative AIHA patients.
Very similar to the study by Baek et al., even in our cohort, all the 6 Evan's syndrome patients had FANA positivity, while none of the FANA negative AIHA patients had Evan's syndrome.7 This was a statistically significant difference. This may point to a more severe and polyspecific autoimmune activation as part of a systemic autoimmune disease. Possibly, the FANA positive Evan's syndrome patient can have a higher underlying autoimmune comorbidity than those who present with AIHA alone. Hence, it may be prudent to evaluate all Evan's syndrome patients for underlying systemic autoimmune diseases.
As far as the hemoglobin and LDH values at presentation were concerned, there was no statistically significant difference between the groups. Moreover, the baseline hemoglobin levels did not correlate with the relapse rates. This may be due to the small number of patients studied. The initial steroid response rates and mean time taken to achieve initial complete response were also not significantly different between the groups. The mean follow-up was similar, however, when relapse rates per month of the patient follow-up was considered, the FANA positive group had a significantly higher rate of relapses. In the FANA negative AIHA patients, 4 patients had a total of 5 relapses in a total of 407 patient-months of documented follow-up. Among the FANA positive patients, there were 20 relapses among 7 patients in a total of 560 patient-months of documented follow-up. The Kaplan Meier curve in Figure one clearly demonstrates an inferior relapse-free survival among ANA positive AIHA patients. This higher rate of relapses in the FANA positive AIHA patients provides an important prognostic implication during decision-making on the modality of treatment offered. The follow-up duration and mean follow-up duration were not statistically significant. Furthermore, the FANA negative AIHA patients had a significantly higher rate of complete remissions which were long- lasting, as evidenced by a total of ten patients who are leading a treatment-free life at the end of 5 years. During this period, one patient in the FANA negative AIHA group had a sudden death at home of an unknown cause. Notably, other than one death and one persisting but stable disease on MMF without complications, all the other primary AIHA patients have achieved remission and are leading a normal life. However, among the 17 FANA positive AIHA patients, only 6 patients achieved a complete remission with a treatment-free life at the end of 5 years. Complications developed in 7 patients, with two developing Class IV lupus nephritis and one patient developing deep vein thrombosis (DVT) and pulmonary embolism. Another 4 patients died, one due to post splenectomy splanchnic thrombosis, two due to sepsis and one due to hemophagocytic syndrome. The death rates were not different between the two groups. The FANA positive cohort had a numerically higher proportion of both death and complications than the FANA negative AIHA group. This was just short of statistical significance with a p-value of 0.555. Thus, the presence of FANA in these patients may suggest higher immunogenicity as part of other autoimmune diseases. The presence of FANA at the time of diagnosis may portend a lesser chance of complete remission and higher risk of relapse. Hence, baseline FANA positivity may help to predict the prognosis for these patients and it may be prudent to initiate a more definitive second-line agent, such as Rituximab, upfront in them. While it cannot be conclusively stated that FANA positivity adversely affects the prognosis in terms of complications as per our study, a larger sample size may alter the results and the FANA positive cases may be associated with higher complication rates.
The mainstay of the treatment of AIHA remains steroids in developing countries, either given as pulse methylprednisolone/dexamethasone or oral prednisolone.2,4,11 After the initial steroid therapy, most patients will require a second-line therapy, as more than 80% of the patients do not achieve complete remission.2,4 The preferred second-line agent has been Azathioprine, which has been slowly replaced by anti-CD20 monoclonal antibody Rituximab, due to better and definitive response rates. Moreover, the drug has become more increasingly affordable due to the availability of generic Rituximab.2,4,12,13,14 Other drugs, such as Danazol,15 Cyclophosphamide, MMF and combination therapies,1,5,16,17 are also used in resistant cases. Once considered second-line therapy, splenectomy is currently performed sparingly, considering the invasiveness of the procedure and post-operative complications, such as sepsis and visceral vein thrombosis and increased fatality rates.1,2
The treatment responses in our cohort were also similar to the available literature. The average number of lines of treatment needed for meaningful recovery was the same in both the groups. Overall steroid response rates were 66.6%. However, only 8 patients had complete responses to steroids alone, with a 29.6% success rate. This is marginally better than in the available literature, in which nearly 80% of the patients in the long run require at least one second line therapy. Our preferred second-line therapy is Azathioprine, given the low cost of the therapy, with relatively few side effects. An overall success rate of 60% with Azathioprine therapy in the entire study population is again in tune with the available literature. The next frequently used drug was Rituximab. The difference in the response rates to Rituximab among the two groups was not statistically significant. Overall, five out of seven patients who received Rituximab achieved a CR, with an overall response rate of 71.42%. However, one patient in the FANA negative group lost his response 18 months after the initial Rituximab therapy and was re-challenged with a second course, with a modest response. No other patient who achieved a CR relapsed after the Rituximab therapy. The response rate we obtained from the use of Rituximab was slightly lower than the response rates that were observed in randomized control trials (RCT).12–14 This is possibly because we used Rituximab only as a third-line agent and not as a first-line treatment. Other treatment options that were used were MMF, Cyclophosphamide and splenectomy. The reported efficacy rate for MMF has been approximately 70%.16 However, in our study both Cyclophosphamide and MMF were used only as salvage therapies when all the other treatment options failed. Furthermore, the number of patients who used either Cyclophosphamide or MMF was too small to draw any conclusions. Splenectomy yielded a 100% immediate response rate in the 4 patients. However, two patients died in the long run, yielding only a 50% long-term success rate. This is again in tune with the available literature, in which splenectomy in patients with autoimmune disorders is more likely to develop into thromboembolic complications.1,2,7,11 With this experience, we now consider splenectomy only as a salvage therapy for patients who have failed to show response to multiple lines of therapies, including multidrug therapy.
As stated in the beginning, AIHA is an uncommon disorder and hence, the literature pertaining to this disease is available mostly as case series.1,2,5–7,10 Prabhu et al. retrospectively studied a group of 33 patients with primary AIHA and looked at treatment responses and compared Warm AIHA with cold agglutinin disease.2 Baek et al. retrospectively analyzed a group of 32 AIHA patients and studied the development of SLE in these patients.7 Barcellini et al. in their analysis of 308 patients (GIMEMA study group) with all forms of AIHA concluded that splenectomized cases, acute renal failure, Evans syndrome and multi-treatment (4 or more lines) are predictive of more severe disease with possible fatality.1 Roumier et al. studied a homogenous group of 60 patients with warm AIHA and investigated the co-existing conditions, such as CLPD and SLE. They also looked at treatment response rates for various treatment modalities and they concluded that the incidence of AIHA is increasing at least at their center and their use of Rituximab is also increasing5. Hantaweepant et al., in their retrospective analysis of 54 Thai patients with primary AIHA, concluded that the relapses are common and majority of the patients need multiple lines of therapy. In their study, the only predictor of more severe disease was strong positive DAT (3+ - 4+). They also observed that males had a better relapse-free survival. We started our study in 2013 and most of the above-mentioned articles were published after that, except for the study by Baek et al.7 Some of the above analyses that could have been possible in our study were not performed, as we did not think along those lines when we started our study. Notably, the mean time to relapse in either group is missing in our data, which could have given a better statistical significance. Also we did not record the interval between the diagnosis and onset of complications. Another drawback of the study is that we could not classify the autoantibodies in these patients, in view of the lack of availability of monospecific reagents in our lab. The strengths of the paper are that it is a prospectively collected data of a homogenous study population from 2013 to 2015 and followed up for the next 5 years. It is a single-center study, imparting more homogeneity and reproducibility to the data. We recruited 32 patients with DAT positivity in a span of only 2 years, as we are a referral center for a population of more than three million . We did not include the Coombs’ Negative AIHA patients in the study. Moreover, patients with only IAT positivity with DAT negativity were also excluded from the study. To our knowledge, ours is probably the first paper looking at the impact of the presence of FANA at the baseline in primary AIHA patients. Readers should note that at the baseline, though seventeen patients had FANA positivity, none of them had any other clinical symptom or sign of any other connective tissue diseases (CTDs), including SLE. Hence, we can safely conclude that our findings are more likely to be driven by underlying FANA positivity alone than the presence of other CTDs. We feel that probably a similar study with a larger pool of patients may show a statistically significant difference in the morbidity and mortality in patients with FANA positive AIHA. The FANA is a simple investigation that can be done at most centers. It has immense value in predicting the outcomes of a rare disease, such as AIHA, at the diagnosis itself. Another large study looking at the baseline FANA, severity of DAT, gender distribution and Evan's syndrome combined would definitely help in establishing a meaningful prognostic sub-classification of primary AIHA patients and thereby aid in better decision-making in the therapeutic modalities.
ConclusionsIt is common to have FANA positivity among patients with AIHA in places where autoimmune diseases are common. Baseline FANA positivity was found to be associated with lower remission rates and higher relapse rates, with possible higher rates of morbidity in AIHA patients. Hence, a baseline FANA at the time of diagnosis of AIHA has to be established for all patients. The presence of FANA will give us a prognostic value and help us in deciding the treatment options.
FundingThis study was not funded by any funding agency.
Dr. Prashantha B did the data analysis and wrote the article, Dr. Aseem Rangnekar collected the data and contributed to the data analysis, Dr. Suchitra Shenoy did the relevant investigations and critical review and Dr. Chakrapani M contributed to the writing of the article and its critical revision. The article was reviewed and approved by all the authors.