Flow Cytometry (FC) is one of the techniques, which allows the identification and characterization of platelets. The detection of absent or reduced expression of the glycoproteins is the main objective of this technique. Abnormalities of glycoproteins lead to hemorrhagic syndromes. Among the main diseases, the Bernard-Soulier syndrome (BSS) and Glanzmann thrombasthenia (GT) stand out. We aimed to show a FC-based platelet assessment test for diagnostic use, which measures the expression of markers in normal patients, and evaluate these markers in patients with platelet disorders.
MethodsWe examined a control group of 41 healthy adults to establish reference values and assess the variability of the relative expression of platelet markers and subsequently compared these findings to those of 30 patients with suspected platelet dysfunctions. We determined the mean fluorescent intensity (MFI) of the expressed parameters by FC using CD41, CD42a, CD42b and CD61 and SSC/FSC platelet-gated cells.
ResultsWe determined our baseline panel of markers and compared them to suspected platelet dysfunctions. Patients with suspected BSS presented increased levels of the MFI for the GPIIIa (CD61) and GPIIb (CD41). They showed significantly reduced levels of the GPIb (CD42b) and GPIX (CD42a). Patients with suspected GT showed normal expression of the GPIX (CD42a), increased expression of the GPIb (CD42b) and reduced levels of the GPIIIa (CD61). In this case, with reduced levels of only one marker, the GPIIb (CD41), values showed normal expression.
ConclusionsWe describe the FC assay to support the diagnosis of different platelet disorders. Our study made it possible to implement a technique that brought benefits to care.
Platelets play an essential role in hemostasis.1 For their characterization, several platelet surface glycoproteins (GPs) were identified as GPIIb, evaluated by the expression of CD41, and non-covalently binds to GPIIIa were recognized by the CD61 marker, making up the GPIIb-GPIIIa complex (CD41/CD61), which mediates aggregation between activated platelets.2,3 The GPIX is identified by CD42a and GPIb, by CD42b, that interacts together with GPV (CD42d), forming the other complex, GPIb-IX-V. When platelets are activated, they express the GP that enables adhesion and aggregation reactions, forming the plug platelet.4 The GPIb-IX-V and GPVI complexes enable binding to the von Willebrand factor (VFW) and subendothelial collagen, respectively, thus being mainly responsible for platelet adhesion to the subendothelium. There is then an increase in the expression of surface adhesion molecules via the GPIIb/IGPIIa complex (CD41/CD61) on the platelet, which mediates aggregation between activated platelets by binding to fibrinogen. Therefore, all these GPs are essential for normal platelet function.1,4
Flow Cytometry (FC) is one of the techniques, which allows the simultaneous identification and characterization of multiple characteristics of resting and activated platelets.1,4 The advantages of using the technique are numerous, including the individual assessment of the extent of platelet activation, detection of platelet subpopulations and quantitative assessment of biomarkers, when compared with other platelet assessment methods that require the isolation of platelets from plasma.4,5 Its disadvantages are the high cost, difficulty in standardizing and obtaining a reference value and observer dependence.6 According to current guidelines, the use of flow cytometry is recommended as an integral component of the investigation of platelet function disorders.7
Parameters, such as forward-scatter (FSC) to determine the size and side-scatter (SSC) to determine granularity, can be used to discriminate cell populations. The unique characteristics of platelets make flow cytometry perfectly suited for their identification.8,9 The detection of absent or reduced expression of the glycoproteins is the main objective of this technique for platelets analysis. Deficiencies and abnormalities in glycoproteins lead to hemorrhagic syndromes that present clinical variability and, among the main associated diseases, the Bernard-Soulier syndrome (BSS) and Glanzmann thrombasthenia (GT) stand out.6
The BSS is an autosomal recessive disease caused by several molecular changes. Patients are prone to bleeding, thrombocytopenia and platelet macrocytosis. These changes produce altered proteins that would have a fundamental role in the stability of the expression of the von Willebrand receptor GPIb/IX/V complex (CD42a/CD42b/CD42d).6,10 Homozygous or compound heterozygous mutations induce loss or severe deficiency of the GPIb-IX complex (CD42a/CD42b) in the platelet membrane, demonstrating a marked reduction of this complex by FC.9,11
The Glanzmann thrombasthenia (GT) is an autosomal recessive thrombocytopathy most often characterized by the absence of platelet aggregation and chronic hemorrhagic dyscrasia caused by deficiency or decrease in the fibrinogen receptor GPIIb-GPIIIa (CD41/CD61).6 Pathogenic mutations impair normal GPIIb/GPIIIa receptor function, weakening platelet aggregation and leading to unstable clot formation and bleeding. Affected patients can range in their symptoms from nearly asymptomatic to bleeding episodes that can vary in intensity and frequency.12,13 Based on their CD41/CD61 levels, patients with GT can be classified into three types, being type I, with absent or extremely low levels (< 5%) of CD41 and CD61, type II, with reduced levels (5–20%), and type III (variant type), with approximately 80 to 100% of the normal levels due to dysfunctional GPIIb/GPIIIa receptors, which may present heterogeneous expression patterns.14,15
Flow cytometry-based approaches to platelet assessment have been used for many years in research settings. The use of FC in diagnosis is already recommended, but the lack of standardization sometimes prevents its use as a diagnostic test.
ObjectiveConsidering the universe of diseases associated with platelet dysfunction that can be identified through the evaluation of the expression of different immunophenotypic markers, it becomes necessary and fundamental, as a first step, to establish these parameters in the control population. In this study, we aimed to (1) show an FC-based platelet assessment test for diagnostic use, which measures the expression of certain markers in normal patients, and to determine reference values in this population and (2) evaluate these markers in patients with platelet disorders and compare them to pre-defined values, aiding in the diagnosis.
MethodsPatientsWe evaluated 41 healthy volunteers at a University Hospital in Porto Alegre in southern Brazil. Peripheral blood samples were collected for immunophenotypic analysis, being processed within two hours after venipuncture. The research project was approved by the Research Ethics Committee of the Hospital and registered under number 2014-0708.
Samples from 30 patients with suspected platelet dysfunctions evaluated by the laboratory routine were included. The inclusion criteria were patients with medical suspicion, abnormal platelet counts, or presence of giant platelets and those who had had a hemorrhage at some point, or had a family history.
Flow cytometryThe technique described below was used to study the expression of platelet antigens. The whole blood sample collected in K2EDTA was previously homogenized for subsequent transfer of 5 µL of its volume to a test tube containing 50 µL of PBS buffer (Laborclin Saline Phosphate buffer). Monoclonal antibodies with previously titrated volumes (10 µL of each marker) were added to individual tubes: CD41a FITC (clone MEM-06, Exbio), CD61 FITC (clone VI-PL2, BD), CD42a FITC (clone ALMA-16, BD), CD42b PE (clone HIP, BD) and CD61 PerCP (clone RUU-PL7F12, BD). The antibodies were incubated for 30 minutes at room temperature and protected from light. After the incubation period, the samples were washed with 2.0 mL of PBS, homogenized by vortexing and first low-speed centrifuged at 900 rpm for 10 minutes to eliminate the leukocyte fraction and, posteriorly, at 2,800 rpm for 10 minutes to obtain the sedimentation of platelets. The supernatant was discarded (platelet-poor plasma) and the platelets were re-suspended with 500 µl of PBS. A total of 30,000 platelet gate events were acquired on the FACSCanto II flow cytometer (BD, São José, CA, USA). For data analysis, the Infinicyt software (Cytognos SL, Salamanca, Spain) was used. The selection of antigens to be evaluated was based on literature recommendations.4
The following gating strategy was used to identify the mean fluorescence intensity (MFI) of a molecule in the platelet population: identification of the platelet population in the parameters Log FSC x SSC and selection of the antigen of interest. From this, the MFI value was estimated within these cells that are included in these two analyses, as shown in Figure 1. As for patients with suspected BSS, the gate is performed on the markers CD41 and/or CD61, followed by FSC vs. SSC, and then the expression of the markers CD42a and CD42b. For the GT the gate is performed on the markers CD42a and/or CD42b, followed by FSC vs. SSC, and then the expression of the markers CD41 and CD61. The flow cytometry data in patients with platelet disorders were compared with the reference values established in the healthy control group in our laboratory.
The MPV (mean platelet volume) and number of platelets were determined by the automatic hematology analyzer.
Statistical analysisAll data are presented as medians with ranges, as not all the data were normally distributed. The non-parametric Kruskal-Wallis test was used to assess the distribution of platelet immunophenotypic markers in the sample and the differences between patients with disorders and healthy individuals. The Statistical Package for the Social Sciences® (SPSS) version 18.0 software was used for analysis, considering a significance of 5%.
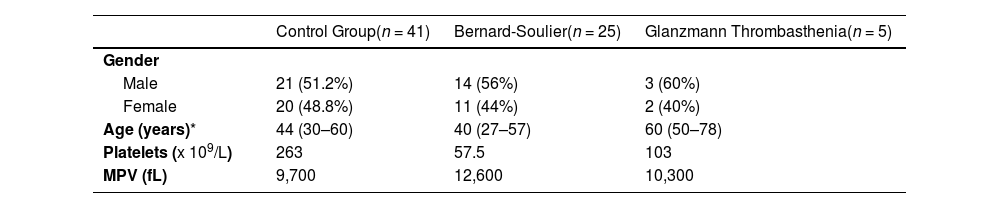
ResultsWe examined a control group of 41 healthy adults of both sexes, with average age of 55 years (45 - 57) to establish standard values and assess the variability of the relative expression of platelet markers. We evaluated the same parameters in patients with suspected platelet dysfunction, 25 with suspected BSS and 5 with GT. The characteristics of the control group and patients with suspected platelet dysfunction are described in Table 1.
Characteristics of patients with suspected platelet disorders and control group.
| Control Group(n = 41) | Bernard-Soulier(n = 25) | Glanzmann Thrombasthenia(n = 5) | |
|---|---|---|---|
| Gender | |||
| Male | 21 (51.2%) | 14 (56%) | 3 (60%) |
| Female | 20 (48.8%) | 11 (44%) | 2 (40%) |
| Age (years)* | 44 (30–60) | 40 (27–57) | 60 (50–78) |
| Platelets (x 109/L) | 263 | 57.5 | 103 |
| MPV (fL) | 9,700 | 12,600 | 10,300 |
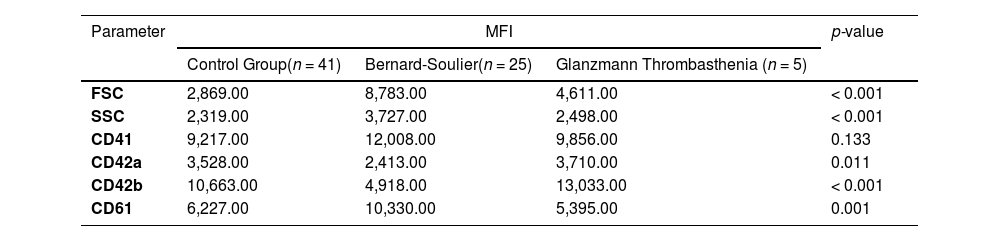
The values of the parameters presented as MFI, both in the control cohort and in patients with suspected platelet dysfunctions, are shown in Table 2.
Mean fluorescence intensity in control group and patients with platelet disorders.
Legend: MFI: mean intensity fluorescence; FSC: Forward Scatter; SSC: Side Scatter.
Subsequently, we aimed to determine whether our basic panel was able to detect characterized experimental conditions and corroborate the diagnosis of these dysfunctions.
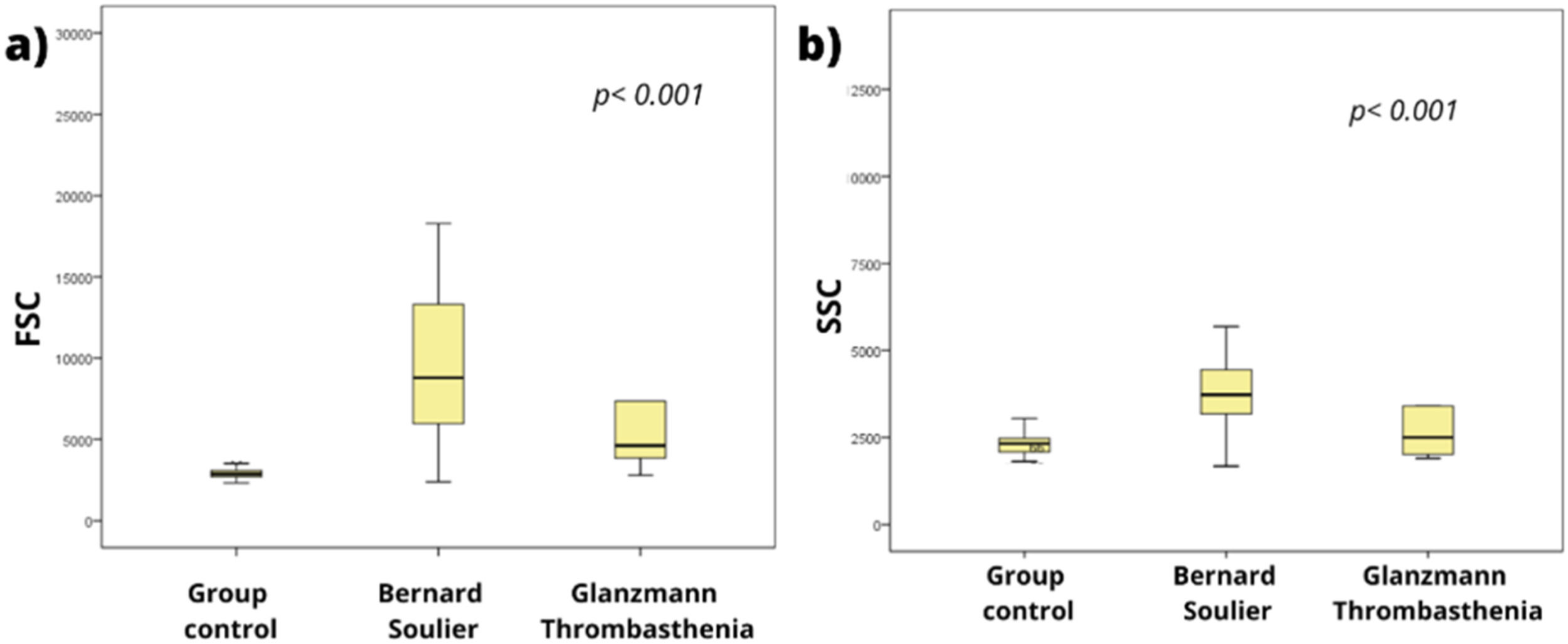
As expected, patients with suspected BSS showed significantly increased values for FSC and SSC, compared to the control group. In turn, patients with suspected GT presented increased FSC (Figure 1).
Group control’ should be changed to “Control Group” in a), b), c), d), e) and f) !!!
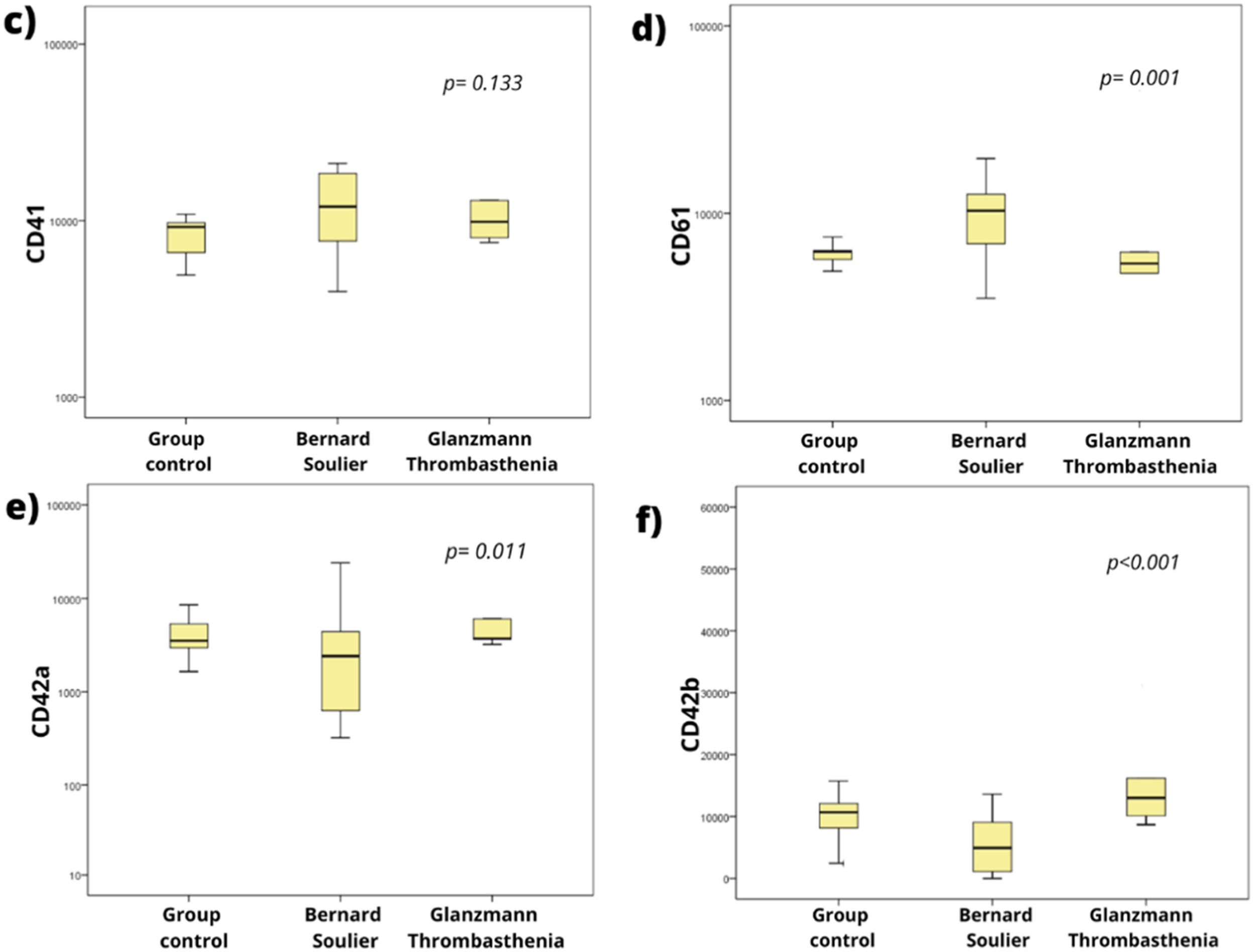
As shown in Figure 2 (c and d), patients with suspected BSS presented increased levels of MFI for GPIIIa (CD61) (p < 0.001) and GPIIb (CD41), but they showed significantly reduced levels of GPIb (CD42b) and GPIX (CD42a) (e and f) compared to the control group (p < 0.001 and p = 0.0011, respectively), corroborating the data in the literature, which characterize the disease as a deficiency or decrease in the GPIb/GPIX complex (CD42b/CD42a) and an increase in the GPIIb (CD41) and/or GPIIIa (CD61) antigens.9-11
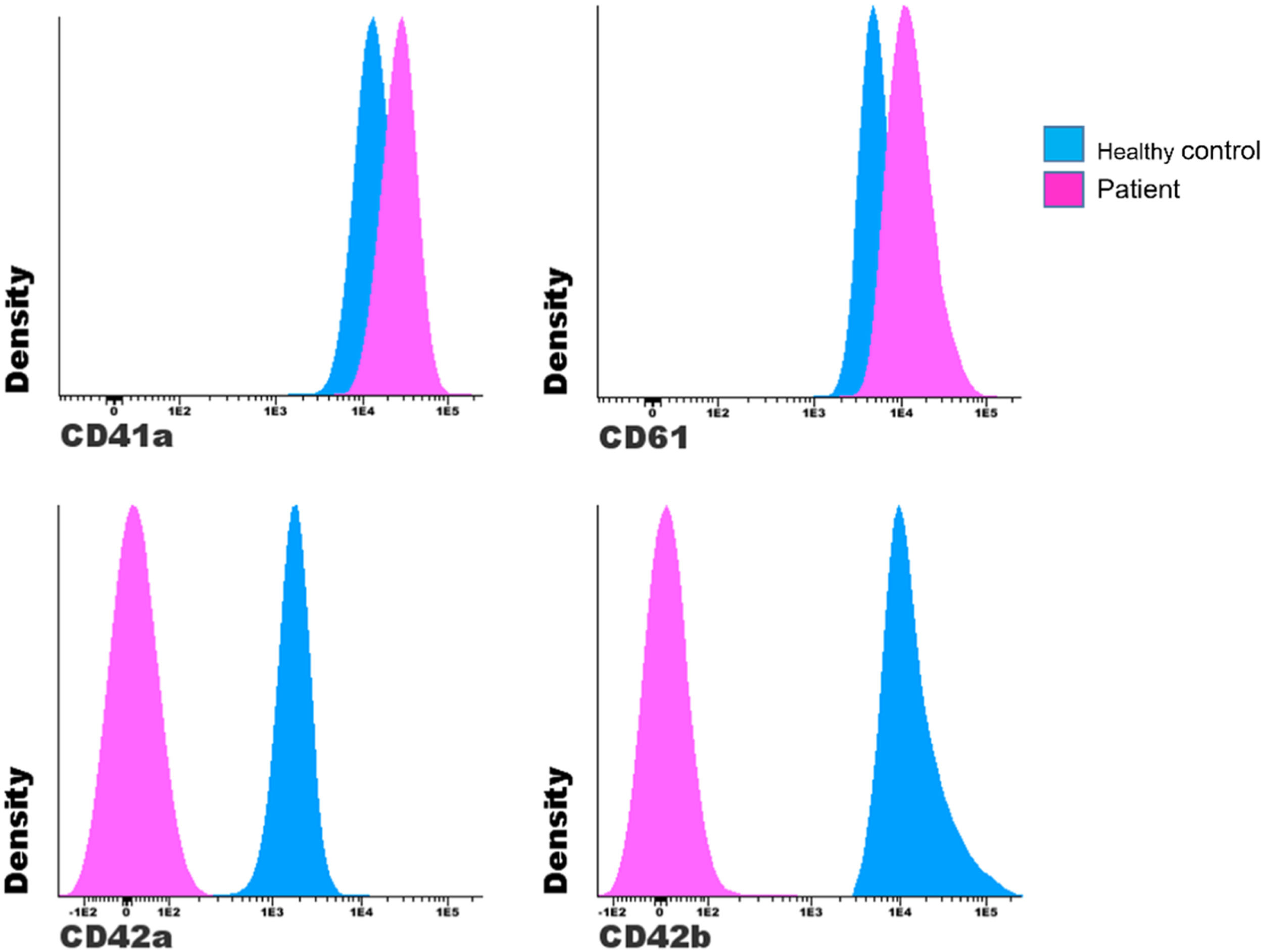
Figure 3 shows an example of a comparison between a patient with suspected Bernard-Soulier and a control. The suspected patient has lower MFI values of the GPIX (CD42a) and GPIb (CD42b) than those of the control, while the GPIIb (CD41) and GPIIIa (CD61) are at values slightly higher than those of the control.
Patients with suspected GT showed normal expression of the GPIX (CD42a), increased expression of GPIb (CD42b) and reduced levels of GPIIIa (CD61), showing expression patterns similar to those described in the literature, which characterizes GT as a deficiency or decrease in one of the markers of the GPIIb/GPIIIa complex (CD41/CD61).14-16 In this case, presenting a heterogeneous pattern, with reduced levels of only one marker. GPIIb (CD41) values showed normal expression, however, this was not significant. We found 3 patients who fit into type II and 2 into type III.
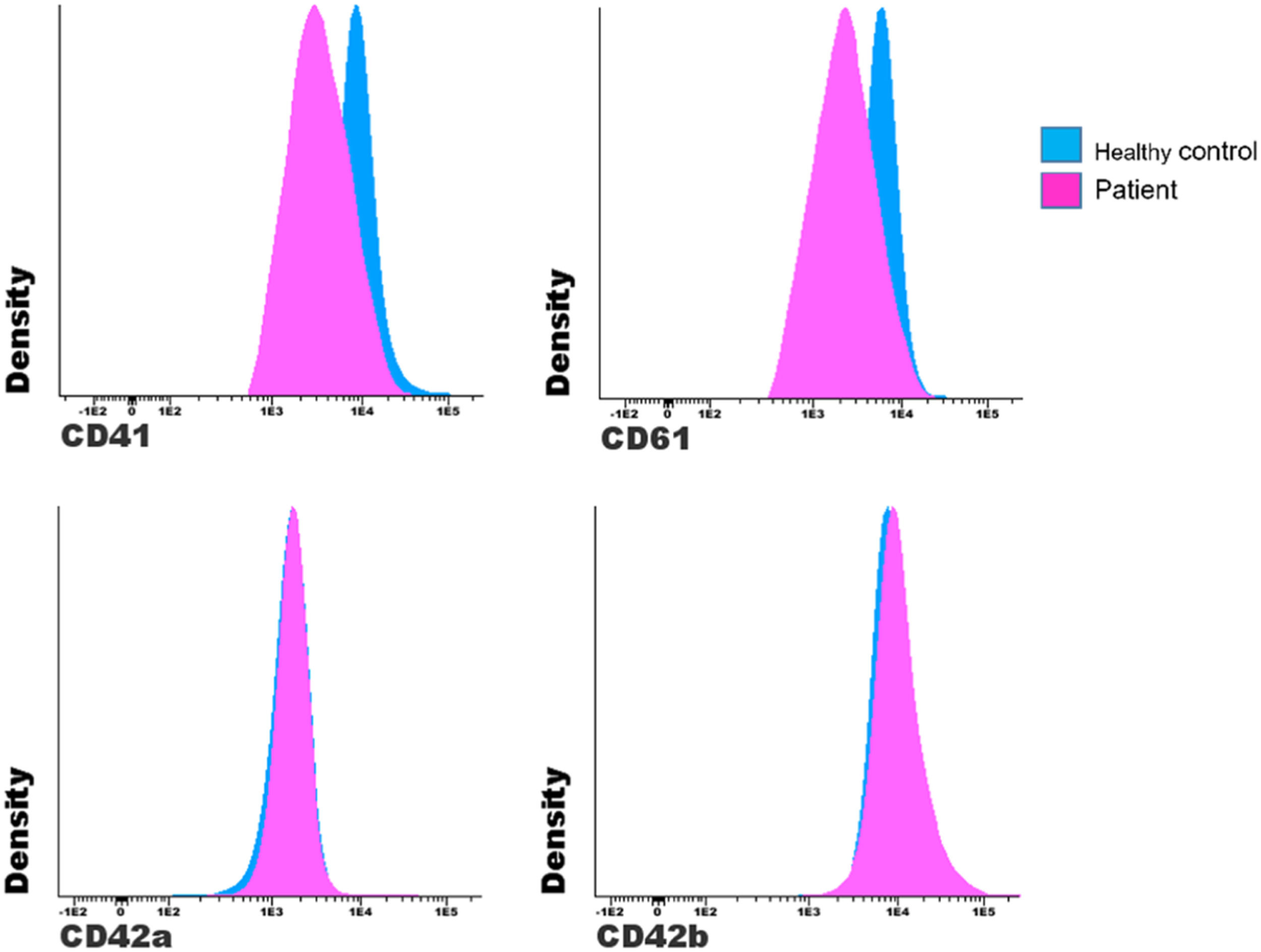
Figure 4 shows an example of a patient with suspected GT who has lower MFI values for markers GPIIb (CD41) and GPIIIa (CD61) and similar values for the GPIX (CD42a) and GPIb (CD42b).
DiscussionWe described the flow cytometry assay to support the diagnosis of different platelet disorders. We standardized and implemented the technique in which the expression profile of platelet-associated immunophenotypic markers was determined in a population of individuals with normal hematology. It was possible to incorporate the test into laboratory research, assisting the study of various pathologies associated with platelet dysfunction using the evaluation of the expression of these markers.
The process of developing a flow cytometry panel should be a judicious step. Rapid detection of the most common platelet disorders is the primary objective of the basic panel. Their model comprises only 5 µl of whole blood and enables the diagnosis of platelet glycoprotein defects, or provides guidelines for certain deficiencies.12 Unfortunately, there are many different protocols available and the detection of platelet function by flow cytometry is sometimes still only applied in research settings due to the lack of standardization across laboratories and instruments.17
With the analyses in our laboratory, it was possible to evaluate 30 patients with suspected platelet dysfunction and contribute to diagnostic elucidation. According to the literature, monoclonal antibodies can be used in the assay to measure the expression of any antigen on the platelet surface, enabling its identification.18 In this study, we only used antibodies aimed at platelet identification, as our objective was to assess their intensity of expression.16,19
In the diagnosis of hereditary deficiencies, the FC provides a quick and simple means for the identification of glycoprotein deficiencies, such as BSS and GT.18 In our suspected BSS group, platelet surface expression of the GPIb-IX (CD42b/CD42a) was markedly reduced in all patients, compared to healthy controls. The GPIIb-GPIIIa (CD41/CD61) complex, on the other hand, presented values above normal, when compared to the control group. The finding of giant platelets in patients with the BSS is consistent with previously reported features of these patients. There should have been additional tests for platelet function, such as bleeding time, platelet aggregation and ATP release. Furthermore, to confirm the proposed diagnosis, other tests, such as clot retraction and mutation analysis, are needed. In the absence of these tests, the correlation with the patient's clinical condition assists in the diagnosis.20
Rubak et al. had already said that it is difficult to identify patients who will have bleeding, as the association between platelet count and risk of bleeding is often not predictable, making the assessment of platelet function essential. He also cited that the FC is independent of the platelet count and, therefore, can be used to diagnose platelet disorders. In his study involving twenty healthy volunteers and five patients diagnosed with different platelet disorders, he developed and evaluated the receptor glycoprotein expression levels. For patients with BSS, he found levels below normal for the GPIb-IX (CD42b/CD42a) and the expression of the GPIa, GPIIb (CD41) and GPIIIa (CD61) above normal.20
Barozzi et al. presented a study with heterozygous and homozygous patients. They showed that in homozygous patients, the glycoproteins of the GPIb-IX (CD42b/CD42a) complex were reduced, compared to healthy controls. The GPIIb-GPIIIa (CD41/CD61) complex glycoproteins were markedly increased, consistent with platelet macrocytosis. The heterozygotes showed a slight reduction in the expression of the GPIb-IX (CD42b/CD42a) complex. In these individuals, the GPIIb-GPIIIa (CD41/CD61) was increased, compared to controls, although to a lesser extent than in homozygous-affected individuals, consistent with their milder platelet macrocytosis.21
The GT is a rare hemostatic disease that leads to severe bleeding disorders with induced or spontaneous bleeding. With regard to quantitative deficiencies, the use in the FC of a single monoclonal antibody directed against the GPIIb (CD41) or GPIIIa (CD61), in which it demonstrates deficiency, is therefore sufficient to support the absence of the complex on the platelet surface.22,23 Mohanty et al. was one of the authors who reported that the GT is divided into three groups: Type-I: patients with less than 5% of GpIIb-GPIIIa (CD41/CD61), Type-II: patients with 5% to 20% of GpIIb-GPIIIa (CD41/CD61) and Type-III (variants) with normal amounts of GpIIb-GPIIIa (CD41/CD61), but functionally inactive.24
The Indian study by Mutreja et al., based on the FC quantification of the GpIIb (CD41) and GPIIIa (CD61) in 51 patients with GT, showed that 47% of their patients were classified as GT type I, 11.8% as GT type II, and 41, 2% had normal expression of the GpIIb (CD41) and GPIIIa (CD61). Patients had significantly lower mean GpIIb (CD41) values, compared to those of the GPIIIa (CD61).14 Hassan et al. evaluated 28 patients in Iraq and revealed that type I was the most common (86%), followed by type III (11%) and type II (3%). In our case, 3 patients fit into type II and 2 into type III. Type III variants usually have dysfunctional receptors with near normal GPIIb/IIIa levels. However, this could be confirmed by more robust studies, with larger numbers of patients and involving analysis of molecular mutations.15,23
Flow cytometry techniques have been used in several laboratory applications, such as quality control of platelet concentrate and immunophenotyping of platelet surface receptors. An additional advantage of flow cytometry, compared to conventional platelet diagnosis, is the small volume of the sample, thus allowing the diagnosis of platelets in infants and young children. However, the technique needs standardization and validation before being implemented in a diagnostic laboratory.25
Our study shows that a standardized approach to the FC has diagnostic potential in patients with suspected bleeding disorders. There are some disadvantages, for example, cytometers are expensive instruments to buy and maintain. The technique itself demands high costs in the maintenance of its analyses, but it will allow great advances in the laboratory and clinical areas. Some care must be taken in the preparation of samples and a dedicated and specialized operator is required, as the flow cytometer requires frequent calibration.25,26
This study allowed for the standardization and implementation of the platelet labeling technique by the FC, showing heterogeneity in the MFI of platelet antigens in healthy individuals, demonstrating the applicability in clinical practice of incorporating the test. It allowed the simultaneous detection of surface antigens in a sensitive and specific way. However, when it comes to a suspected change in expression intensity and not just the total absence of the marker, clones and fluorescence must be controlled and a control sample must always be analyzed along with the patient in question.17 It then became necessary for each laboratory to standardize its own technique, as it became clear that there are variations in the test, which must be investigated according to the situation of each laboratory and availability of markers and reagents.
ConclusionThese results were important to know the intensity profile of these markers in healthy individuals, allowing applications in patients with platelet disorders, aiding in diagnosis and contributing to the laboratory routine. These analyses can be implemented in clinical practice to improve diagnosis for patients with suspected platelet disorders. The work is part of a larger project involving platelet diagnosis. Therefore, future studies will be carried out to evaluate molecular alterations and obtain genetic confirmation.
The study received financial support from the Research and Event Incentive Fund of the Hospital de Clínicas de Porto Alegre (FIPE-HCPA).