The purpose of this study was to evaluate the effectiveness of mature red cell and reticulocyte parameters under three conditions: iron deficiency anemia, anemia of chronic disease, and anemia of chronic disease associated with absolute iron deficiency.
MethodsPeripheral blood cells from 117 adult patients with anemia were classified according to iron status, and inflammatory activity, and the results of a hemoglobinopathy investigation as: iron deficiency anemia (n=42), anemia of chronic disease (n=28), anemia of chronic disease associated with iron deficiency anemia (n=22), and heterozygous β thalassemia (n=25). The percentage of microcytic red cells, hypochromic red cells, and levels of hemoglobin content in both reticulocytes and mature red cells were determined. Receiver operating characteristic analysis was used to evaluate the accuracy of the parameters in differentiating between the different types of anemia.
ResultsThere was no significant difference between the iron deficient group and anemia of chronic disease associated with absolute iron deficiency in respect to any parameter. The percentage of hypochromic red cells was the best parameter to discriminate anemia of chronic disease with and without absolute iron deficiency (area under curve=0.785; 95% confidence interval: 0.661–0.909, with sensitivity of 72.7%, and specificity of 70.4%; cut-off value 1.8%). The formula microcytic red cells minus hypochromic red cells was very accurate in differentiating iron deficiency anemia and heterozygous β thalassemia (area under curve=0.977; 95% confidence interval: 0.950–1.005; with sensitivity of 96.2%, and specificity of 92.7%; cut-off value 13.8).
ConclusionThe indices related to red cells and reticulocytes have a moderate performance in identifying absolute iron deficiency in patients with anemia of chronic disease.
New automated blood cell analyzers can provide information about individual cell characteristics, including the hemoglobin content of reticulocytes and mature red blood cells, and percentages of microcytic red cells and hypochromic red cells. These new parameters have been used in the diagnosis of iron deficiency anemia (IDA), β thalassemia (β-thal) carriers,1–3 and anemia of chronic disease (ACD).4,5 The differentiation between these three conditions is very important as the clinical approach is unique to each particular condition.
As reticulocytes have a normal life span of one to two days, information concerning the hemoglobin content of young red cells is a good measurement of the iron availability and an early marker of iron deficient erythropoiesis.6 Reticulocyte hemoglobin equivalent (Ret-He) reflects real-time information on the synthesis of young red cells in the bone marrow. Other available parameters are the percentage of red cells with Hb content equivalent to or less than 17pg (%HypoHe), and the percentage of red cells with a volume of less than 60fL (%MicroR),1 which corresponds to a sub-population of mature red cells exhibiting evidence of insufficient iron content.
A mathematical formula using %MicroR and %HypoHe (MHe), proposed by Urrechaga et al.,7 tested discriminant indices in healthy individuals, β-thal and IDA patients; its sensitivity was 97.4% and specificity was 97.1% in differentiating β-thal from mild IDA.
Anemia associated with chronic inflammation, infection or malignancy is the most common anemia in hospitalized patients. Although stainable iron is present in the bone marrow, elevated levels of inflammatory cytokines interfere in erythropoiesis, leading to a hyporegenerative anemia and defective iron incorporation into red cell progenitors. Reduced concentrations of circulating iron and normal or increased iron stores characterize a state of functional iron deficiency.8
Anemia of inflammation can be associated with absolute iron deficiency (ADC combi), generally in patients with inflammatory disease and chronic blood loss. Differentiation between ACD and ACD combi is clinically important, but in the clinical practice this differentiation is difficult when using conventional biomarkers such as ferritin concentration and transferrin saturation.9 The soluble transferrin receptor/log ferritin ratio may be useful in distinguishing ACD from ACD combi.10
The aim of the study was to analyze the effectiveness of new laboratory parameters related to mature red blood cells and reticulocytes in differentiating three conditions related to iron deficiency: IDA, ACD and ACD combi. Indeed, the performance of the parameters will be tested to distinguish IDA from β-thal, two common causes of microcytic anemia.
MethodsThis project was approved by the Ethics Committee of the Faculdade de Ciências Médicas da Universidade Estadual de Campinas (UNICAMP), São Paulo, Brazil. All samples were selected from routine blood collections and so informed consent was waived.
Peripheral blood samples from 117 adult patients with anemia (Hb<12.0g/dL for women and Hb<14.0g/dL for men) were selected from the routine workload. Blood analysis had been requested by general practitioners mostly to investigate anemia.
Patients were classified according to iron status analysis (commercial kits from Roche Diagnostics, Germany): IDA when serum iron (SI) levels were <45mg/dL for men and <30mg/dL for women, transferrin saturation <15% and serum ferritin <30μg/L for men and <13μg/L for women.
Patients were classified as ACD when SI levels were normal or less than normal (40–160mg/dL and 30–160mg/dL for men and women, respectively), transferrin saturation was normal or less than normal (30–50%), serum ferritin levels were normal or high (30–400μg/L and 13–150μg/L for men and women, respectively) and C-reactive protein >5mg/dL (Tina-Quant C-Reactive Protein, Roche Diagnostics, Germany).
Soluble transferrin receptor (sTfR) levels (Roche Diagnostics, Germany) were measured in all samples, and the sTfR/log ferritin ratio was used to identify iron deficiency in patients with ACD. Patients with ACD showing sTfR/log ferritin >2.06 or sTfR >3.71μg/mL (cut-off values indicative of iron deficiency in our laboratory) were classified as ACD combi.
Twenty-six patients had diagnoses of β-thal according to the level of hemoglobin A2 determined by high performance liquid chromatography (HPLC-Variant II – Hemoglobin Testing System, Bio-Rad Laboratories, Inc., CA, USA).
Patients with β-thal associated with other kinds of anemia, patients with reticulocytosis or pancytopenia, individuals that had received transfusions within the previous three months, and patients on iron replacement therapy were excluded from the study.
A control group was composed of apparently healthy individuals with no clinical signs or symptoms of disease, including acute inflammatory/infection, and with normal hematologic findings, and C-reactive protein <5mg/L. The healthy individuals were students or laboratory staff, all of whom had donated blood samples on a voluntary basis.
Determination of red cell and reticulocyte parameters was performed using a Sysmex XE-5000 automated hematological analyzer (Sysmex, Kobe, Japan), which provided the following parameters: Ret-He, %MicroR, hemoglobin content of red cells obtained from optical counting (RBC-He), and %HypoHe. The MHe index was calculated as %MicroR−%HypoHe.7
The Mann–Whitney test was applied to compare the groups. A receiver operator characteristic (ROC) curve was used to evaluate the accuracy of the parameters to differentiate between the different types of anemia. The level of significance was set at a p-value<0.05. Data were analyzed using SPSS for Windows, Version 13.0 (SPSS Inc., Chicago, IL, USA).
ResultsAccording to adopted criteria, individuals were classified as: IDA=42 patients, β-thal=25 individuals, ACD combi=22 patients, ACD=28 patients and control group=54 individuals.
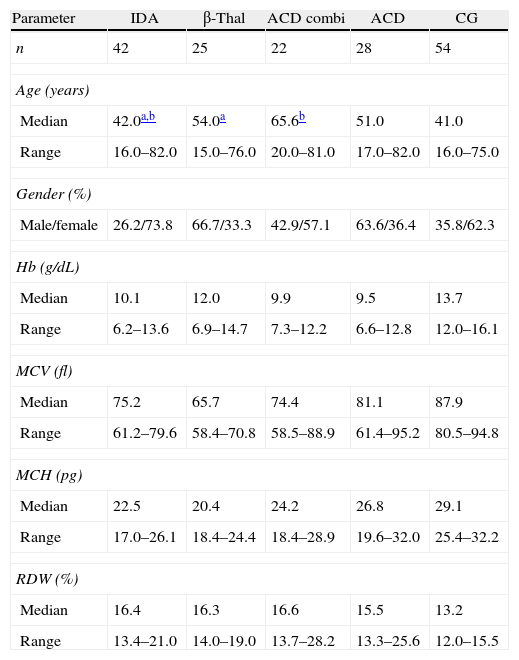
Table 1 describes the demographic characteristics and laboratorial data of the patients and control group, and Table 2 shows the iron status measurements used to classify the patients in the different groups.
Demographic characteristics and hematological data for patients and control group.
| Parameter | IDA | β-Thal | ACD combi | ACD | CG |
| n | 42 | 25 | 22 | 28 | 54 |
| Age (years) | |||||
| Median | 42.0a,b | 54.0a | 65.6b | 51.0 | 41.0 |
| Range | 16.0–82.0 | 15.0–76.0 | 20.0–81.0 | 17.0–82.0 | 16.0–75.0 |
| Gender (%) | |||||
| Male/female | 26.2/73.8 | 66.7/33.3 | 42.9/57.1 | 63.6/36.4 | 35.8/62.3 |
| Hb (g/dL) | |||||
| Median | 10.1 | 12.0 | 9.9 | 9.5 | 13.7 |
| Range | 6.2–13.6 | 6.9–14.7 | 7.3–12.2 | 6.6–12.8 | 12.0–16.1 |
| MCV (fl) | |||||
| Median | 75.2 | 65.7 | 74.4 | 81.1 | 87.9 |
| Range | 61.2–79.6 | 58.4–70.8 | 58.5–88.9 | 61.4–95.2 | 80.5–94.8 |
| MCH (pg) | |||||
| Median | 22.5 | 20.4 | 24.2 | 26.8 | 29.1 |
| Range | 17.0–26.1 | 18.4–24.4 | 18.4–28.9 | 19.6–32.0 | 25.4–32.2 |
| RDW (%) | |||||
| Median | 16.4 | 16.3 | 16.6 | 15.5 | 13.2 |
| Range | 13.4–21.0 | 14.0–19.0 | 13.7–28.2 | 13.3–25.6 | 12.0–15.5 |
IDA: iron deficiency anemia; β-thal: heterozygous beta thalassemia; ACD combi: anemia of chronic disease associated with absolute iron deficiency; ACD: anemia of chronic disease; CG: control group; Hb: hemoglobin; MCV: mean cell volume; MCH: mean cell hemoglobin; RDW: red cell distribution width.
Biochemical data.
| Parameters | IDA | β-Thal | ACD combi | ACD | CG |
| n | 42 | 25 | 22 | 28 | 54 |
| SI (mg/dL) | |||||
| Median | 24.5 | 94.0 | 23.5 | 28.0 | 100.0 |
| Range | 7.0–73.0 | 49.0–224.0 | 12.0–99.0 | 9.0–98.0 | 49.0–185.0 |
| TS (%) | |||||
| Median | 6.1 | 31.9 | 9.2 | 11.6 | 29.5 |
| Range | 1.5–15.5 | 22.4–68.2 | 4.5–25.7 | 3.0–42.2 | 17.6–50.1 |
| SF (μg/L) | |||||
| Median | 8.2 | 224.5 | 254.5 | 163.7 | 59.6 |
| Range | 3.2–23.4 | 66.8–1619.0 | 27.8–2000.0 | 42.1–2000.0 | 19.7–407.9 |
| sTfR (μg/mL) | |||||
| Median | 9.4 | 7.4 | 7.0 | 2.7 | 2.4 |
| Range | 1.4–28.8 | 2.6–25.5 | 3.5–22.4 | 0.5–6.2 | 1.6–3.8 |
| sTfR/log ferritin | |||||
| Median | 10.4 | 2.6 | 3.4 | 1.1 | 1.3 |
| Range | 1.9–40.9 | 1.3–10.9 | 1.3–9.6 | 0.2–3.8 | 0.8–2.2 |
IDA: iron deficiency anemia; β-thal: heterozygous beta thalassemia; ACD combi: anemia of chronic disease associated with absolute iron deficiency; ACD: anemia of chronic disease; CG: control group; SI: serum iron, TS: transferrin saturation; SF: serum ferritin; sTfR: soluble transferrin receptor.
As expected the β-thal group had the highest %MicroR (Table 3). The %HypoHe was also higher in the β-thal group when compared to other groups, except for IDA patients. However, as the microcytic cells were more abundant in the β-thal group, when these two parameters were associated in the MHe index, the difference became more evident and statistically significant.
Reticulocyte and red cell indices for patient and control groups.
| Parameters | IDA | β-Thal | ACD combi | ACD | CG |
| n | 42 | 25 | 22 | 28 | 54 |
| Ret-He (pg) | |||||
| Median | 25.2 | 23.0 | 26.0b | 29.7c | 35.1 |
| Range | 16.9–32.6 | 21.1–30.2 | 19.2–37.0 | 21.0–39.5 | 31.0–39.2 |
| RBC-He (pg) | |||||
| Median | 23.4a | 21.9 | 26.4b | 28.7c | 31.8 |
| Range | 16.7–35.3 | 18.8–26.3 | 19.5–30.5 | 20.8–34.7 | 28.0–34.2 |
| HypoHe (%) | |||||
| Median | 6.2 | 9.4 | 4.4b | 1.4c | 0.2 |
| Range | 0.4–44.5 | 1.4–20.4 | 1.1–31.5 | 0.3–15.3 | 0.1–0.8 |
| MicroR (%) | |||||
| Median | 13.5a | 29.2 | 14.2b | 5.55c | 1.4 |
| Range | 0.4–48.5 | 19.3–54.2 | 0.6–59.0 | 1.2–48.9 | 0.5–4.0 |
| MHe | |||||
| Median | 5.8a | 23.0 | 7.2b | 4.05c | 1.15 |
| Range | −9.8 to 22.2 | 8.1–34.6 | 0.0–27.5 | 0.5–36.3 | 0.3–3.7 |
IDA: iron deficiency anemia; β-thal: heterozygous beta thalassemia; ACD combi: anemia of chronic disease associated with absolute iron deficiency; ACD: anemia of chronic disease; CG: control group; Ret-He: reticulocyte hemoglobin content; RBC-He: red blood cell hemoglobin content; HypoHe: percentage of hypochromic red cells; MicroR: percentage of hypochromic red cells; MHe: MicroR−HypoHe index.
Mann–Whitney test was applied for comparison between groups.
The Mann–Whitney test showed no significant difference between the IDA and ACD combi groups in respect to all parameters. When the ACD and ACD combi groups were compared, the RBC-He and Ret-He were significantly lower for the ACD combi group (p-value=0.016 and p-value=0.003, respectively). Meanwhile, the %MicroR, %HypoHe and the value of MHe were significantly higher in the ACD combi group (p-value=0.001, p-value=0.003 and p-value=0.014, respectively).
Although the ACD group had sTFR/log ferritin values below the cut-off limit indicative of iron deficiency, the Ret-He and RBC-He values were significantly reduced when compared to the control group. However, the %HypoHe, %MicroR and MHe were higher (p-value<0.001 for all).
The best test to differentiate IDA from β-thal was the MHe index (area under curve – AUC: 0.977; 95% confidence interval [95% CI]: 0.950–1.005). Values below the cut-off of 13.8 showed a sensitivity of 96.2% and specificity of 92.7% in identifying IDA patients. A good performance was seen for the %MicroR (AUC: 0.886; CI 95%: 0.810–0.963), and values <25.0% gave sensitivity of 84.6% and specificity of 78.0% in detecting iron deficiency.
When the ROC curve was applied to the ACD and ACD combi groups, the best performance was seen with the %HypoHe parameter, although with a moderate AUC value (AUC: 0.785; 95% CI: 0.661–0.909; sensitivity 72.7%, and specificity 71.4%; cut-off: 1.8%).
The best parameter to distinguish IDA from ACD was the %HypoHe (AUC: 0.835; 95% CI: 0.737–0.933). A value for %HypoHe<2.45% had a sensitivity of 75.4% and specificity of 70.4% in identifying ACD. The AUCs were lower for RBC-He (AUC: 0.809; 95% CI: 0.696–0.922), Ret-He (AUC: 0.780; 95% CI: 0.661–0.899), and %MicroR (AUC: 0.785; 95% CI: 0.662–0.908).
The capacity of the tests in discriminating IDA from ACD combi was not satisfactory as the AUC was lower than 0.700 for all parameters.
DiscussionThe diagnostic performance of reticulocyte parameters has been tested by many authors, especially for the diagnosis of iron deficiency in patients submitted to dialysis.6,10,11 Measurement of the reticulocyte content is helpful in detecting the earliest stages of iron deficiency, prior to the development of anemia.6,12,13 The reduction in reticulocyte hemoglobin has been observed in other conditions besides iron deficiency, such as in hemoglobinopathies.14,15
The effectiveness of using reticulocyte parameters to diagnose IDA and ACD has been tested in other studies.2,8,16 In a recent study with geriatric patients, the authors concluded that Ret-He does not perform better than the classic indices, such as mean cell hemoglobin and mean cell hemoglobin concentration in differentiating between IDA and ACD.5 Our results show that, although the Ret-He value was lower in IDA than ACD, the accuracy of the test to distinguish both types of anemia was moderate, and lower than using the %HypoHe. A potential utility of Ret-He was demonstrated in a study with patients with chronic rheumatic disease and anemia. The predictive value of Ret-He was tested in response to oral iron therapy, and according to the authors, the findings support the role of Ret-He as a marker for iron responsiveness.17
Additional red blood cell parameters, besides Ret-He, have been tested, such as the %MicroR, and %HypoHe, and other indices generated by combining them.18 The results regarding the differentiation between IDA and β-thal are promising, although the optimum cut-off point has varied according to study population and criteria adopted to classify anemia.18,19 The MHe index was first proposed and tested by Urrechaga et al.16 The performance of this index was better (sensitivity 98.0% and specificity 95.9) than other published indices. The authors suggest that samples with MHe values >11.5 can be chosen for further analysis to confirm the diagnosis of β-thal. These data are coincident with our results. The MHe index showed the best performance in discriminating IDA from β-thal, although the cut-off value is different from the value described by Urrechaga et al.,16 probably because we did not separate patients according to the severity of anemia.
As far as we know, no other reports exist about the efficiency of other red cell parameters in patients with anemia of inflammation associated with absolute iron deficiency. The clinical utility of the determination of the %Hypo has long been recognized in differentiating between iron-deficient and iron-sufficient patients with chronic kidney disease who receive erythropoietin stimulating agents.20
The sTfR/log ferritin ratio values were calculated to identify iron deficiency in patients with ACD. The measurement of sTfR has been considered a good indicator of functional iron status as it does not suffer the influences of systemic inflammation unlike SI, transferrin, and ferritin measurements.21,22 Thus, when there is a reduction of functional iron, transferrin receptor synthesis is stimulated, as was observed in our results. Iron deficiency is characterized by an increase in sTfR levels and low ferritin values, while in anemia of inflammation, transferrin receptor levels are only slightly affected and serum ferritin is greatly increased.23 In the clinical practice this differentiation is important because iron supplementation is beneficial for ACD combi, but may be deleterious for ACD patients. According to our results the best parameter to distinguish ACD combi from ACD was the %HypoHe even though the sensitivity and specificity were moderate, followed by %MicroR. It is interesting to note that there were no differences between the IDA and ACD combi groups for any test, as opposed to differences observed when ACD was compared with IDA. In fact, the absolute iron deficiency associated to ACD increases the number of microcytic and hypochromic red cells, developing cell features similar to IDA. ACD patients showed evidence of reduced iron availability for erythropoiesis, but the disturbance of the iron metabolism in functional iron deficiency was less remarkable than in the association of ACD with IDA.
In practical terms the incorporation of new cellular indices can speed up diagnosis of IDA, β-thal and ACD, and consequently target more quickly and more precisely the subsequent confirmatory exams in order to introduce the appropriate treatment. On the other hand, the difficulty in identifying absolute iron deficiency in patients with inflammatory conditions remains. Therefore, the challenge persists, and other studies are needed to find a parameter with value in clinical decision making.
Conflicts of interestThe authors declare no conflicts of interest.