This study was performed to evaluate the degree of 3-day chemotherapy-induced nausea and vomiting (CINV) in children with cancer who received highly emetogenic chemotherapy (HEC) to ascertain the efficacy of aprepitant single-dose on dayL 1 plus granisetron and dexamethasone (DEX).
MethodsThis clinical trial study was conducted on 120 patients in the age range of 5 to 18 years old who received chemotherapy. Patients were divided into two groups; Group A received aprepitant at 125 mg/kg on day 1 orally, followed by 80 mg/kg daily on days 2 and 3 and Group B received a single dose of aprepitant 125 mg/kg on day 1 orally and placebo on days 2 and 3. All groups received granisetron 3 mg/m2 on day 1 and DEX on days 1 to 3. The primary and secondary endpoints were to evaluate the proportion of patients with acute, delayed and overall CINV within each group.
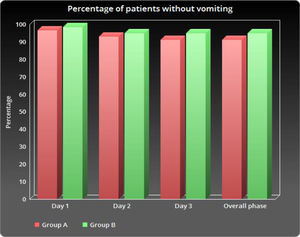
ResultsThere were no significant differences between the two groups for vomiting, nausea or the use of rescue therapy. The number of patients without vomiting on day 1 was similar in both groups (96.5% vs. 98.3%, respectively; p = 0.848).
ConclusionAccording to the results of this study, a single dose of aprepitant 125 mg/kg was as effective as administering three doses of aprepitant on 3 days. Therefore, the use of a single dose of aprepitant in combination with other standard treatment regimens to prevent CINV in children who received HEC was safe and efficacious and can be beneficial.
Chemotherapy-induced nausea and vomiting (CINV) are one of the distressful effects of chemotherapy, which may result in the patient's anxiety and depression. These two symptoms are the first and second most-feared side effects of chemotherapy. The pathophysiology of CINV is highly complicated and it will significantly disturb the life quality of patients with cancer.1,2 Combination therapy of 5-hydroxytryptamine-3 (5-HT3) receptor antagonists with dexamethasone (DEX) has a significant effect on acute CINV.3 The most common standard therapy for CINV control in children is the use of antagonists of receptor 5-HT3 in combination, with or without a corticosteroid.4,5
The 5-HT3 receptor antagonists include dolasetron, ondansetron, palonosetron, tropisetron and granisetron.6 Granisetron (Kytril) is a selective 5-HT3 receptor antagonist which is recommended by clinical practice guidelines as an effective and potent antiemetic drug.7 Application of antagonists of receptor 5-HT3, along (Shouldn't this be “prolonged”???) with DEX, can improve CINV in the acute stage, but the delayed CINV has remained an important clinical issue.8,9
Aprepitant is one of the drugs approved by the FDA for the control of acute and chronic CINV which acts as a neurokinin 1 (NK1) ‐receptor antagonist.10 Regarding its pharmacokinetics, it requires lower doses of DEX, which has distinguished it from other groups. The side effects of this group are also negligible, compared to other groups.11 Aprepitant has been approved for use in combination with a selective 5‐HT3–receptor antagonist, such as granisetron, in adults.12 These patients, undergoing highly emetogenic chemotherapy (HEC) with uncontrolled CINV, usually require longer hospitalization, but the combined effects of granisetron and aprepitant in the prevention of CINV in children undergoing chemotherapy have been less studied. This clinical trial was performed to evaluate the degree of 3-day CINV in children with cancer undergoing HEC to ascertain the efficacy of aprepitant single-dose on day 1 plus granisetron and DEX versus aprepitant on days 1 to 3, in combination with granisetron and DEX.
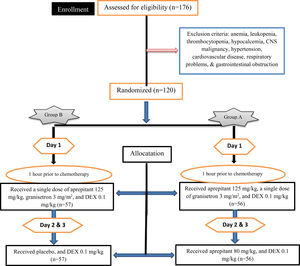
Methods and materialsStudy populationA randomized, triple-blind clinical trial was performed, enrolling 176 children in the age range of 5 to 18 years old who were chemotherapy candidates for acute lymphoblastic leukemia, rhabdomyosarcoma, Wilms tumor, acute myeloid leukemia, osteosarcoma and hepatocellular carcinoma at the Amir-Kabir Hospital in Arak, Iran. This study was a retrospective observational study over a 12-month period.
These patients were scheduled to receive an HEC (Doxorubicin, Cyclophosphamide, Vincristine, Cisplatin, Dacarbazine, Vinblastine, Bleomycin and Actinomycin D). The enrollment of participants, as well as the allocation and allocation of intervention instructions, was performed by a pediatrician and researcher. Randomization was performed using random number generation in Excel software; each patient had an identical number (ID) based on the visiting sequence and these IDs were entered into the Excel software. Thereafter, the “RAND” function was included in another column and the RAND function was entered for each patient, which automatically generated random numbers in Excel rows. After applying the ascending sorting, the random numbers and the patient IDs positions were changed randomly. The sorted IDs were divided into two groups. This study was approved by the Ethics Committee of Arak University of Medical Sciences (ethical committee code number: IR.ARAKMU.REC.1396.46). The trial was registered at the Iranian Registry of Clinical Trials as IRCT20100127003210N19. The inclusion criteria included the following: the presence of cancer confirmed by the molecular assays and histopathological examination; patients who had not received chemotherapy or radiotherapy for 6 months prior to the study; patients who did not have other clinical features, such as anemia, leukopenia and thrombocytopenia; patients who had other disorders causing CINV, such as hypocalcemia, central nervous system malignancy and gastrointestinal disorders; patients who did not have uncontrolled diabetes; patients with normal liver and kidney function, and; patients with normal immune systems. Furthermore, patients with hypertension, fever, severe infection, tinnitus and cardiovascular disease and patients with respiratory problems were excluded from this study. Based on the type of study, the sample size was calculated using Pass 11 software with type I (a) error left at 5 % and type II (b) error left at 20 %, and study power of 90%. The mean ± standard deviation (SD) was 10.6 ± 0.6 in group A and 11.3 ± 0.4 in group B. The sample size of 51 patients was determined for each group, but considering the possibility of some patients missing, the minimum sample size of 57 was considered.
Therapeutic protocolEligible children received HEC, including cisplatin, anthracycline and cyclophosphamide combinations (e.g., fluorouracil, epirubicin, doxorubicin and docetaxel). Random numbers generated on the computer were used for randomization. For all principal researchers, patients, and study evaluators, except for the experienced trained physician of the Amir-Kabir Hospital, randomization was blinded (triple-blinded) to the study. Study bias, through masking the patients and study evaluator, was reduced. After obtaining informed written consent and based on the anti-emetic treatment regimen, patients were divided into group A, 60 patients who received aprepitant at 125 mg/kg on day 1 orally, followed by 80 mg/kg daily on days 2 and 3, a single dose of granisetron at 3 mg/m2 intravenously (i.v.) on day 1, half an hour before HEC, along with 0.1 mg/kg of DEX on day 1 and 0.1 mg/kg daily on days 2 and 3, 1 hour before HEC and group B, 60 patients who received a single dose of aprepitant 125 mg/kg on day 1 orally and placebo on days 2 and 3, a single dose of granisetron 3 mg/ m2 (i.v.) on day 1, along with 0.1 mg/kg of DEX orally on day 1, and 0.1 mg/kg daily on days 2 and 3, 1 hour before HEC. The shape and size of the placebo drugs were similar to those of the original drugs. Patients were asked not to discontinue or alter medication during the study. Information on the patient chemotherapy history (naive or non-naive to chemotherapy) was extracted from their records. There had been a 6-month chemotherapy-free period since the prior chemotherapy of the patients who had a history of chemotherapy and these patients did not have CINV at the time of the study. Figure 1 summarizes the doses and administrative sequences of antiemetics.
Evaluation of CINVA single emetic episode was considered as at least one episode of vomiting, separated by less than a 5-minute interval, during the current chemotherapy cycle. Nausea is a feeling in the stomach that may be accompanied by vomiting. The severity of nausea was assessed by using a visual analogic scale (VAS), ranging from 0 to 100 mm (VAS < 5mm = least severe [no nausea], VAS < 25mm = no significant nausea, 100 = most severe). Patients were asked to record in a diary the number of emetic episodes and the severity of nausea during the 72 hours following chemotherapy. A coordinator had conversations with patients during these three days to ensure that patients recorded all cases of vomiting and nausea correctly. The primary efficacy endpoint was the percentage of patients with a complete response (CR). The CR was defined as no emesis, no significant nausea (or mild nausea, VAS < 25) and no use of rescue therapy during day 1 (acute phase) following the chemotherapy cycle. Secondary efficacy endpoints included CR for the days 2 and 3 (delayed phase) and overall phases (days 1 - 3) after the chemotherapy cycle and no emetic episodes (vomiting events) and nausea (mild nausea), no use of rescue antiemetic therapy (prescribed for the treatment of chemotherapy-induced emesis that did not respond to the initial prophylactic treatment) within the three- day study period, time to first breakthrough antiemetics administered, number of vomiting episodes and the severity of nausea (mild, moderate or severe) within three days.
Statistical analysisStatistical analyses were performed using the SPSS version 18 (Inc., Chicago, IL, USA). A p < 0.05 was considered statistically significant. Data were expressed as mean ± SD for numerical variables. The two groups were compared using Pearsonʼs χ2 test (or Fisherʼs exact test) for categorical variables and the Student t-test for continuous variables.
ResultsPatient findingsOf a total of 120 patients with cancer, 68 patients (56.7%) were male and 52 patients (43.3%) were female. The mean ± SD age of the patients at diagnosis was 9.47 ± 3.05 years (range 5 - 18 years). During the study, 7 patients dropped out and were excluded from the study due to not receiving chemotherapy or antiemetic drugs (4 patients in group A, 3 patients in group B). As a final result, 113 patients remained in the study. Two treatment groups received a single dose of granisetron and DEX, whereas 56 patients received the 3-day aprepitant regimen orally and 57 patients received a single dose of aprepitant on day 1 and placebo on days 2 and 3. The clinical and demographic findings of all patients are shown in Table 1. No statistically significant differences were found between the clinical and demographic findings of the patients in the two groups (p > 0.05). There were no statistically significant differences in age, gender, weight, height, body mass index, history of CINV due to pregnancy, history of alcohol consumption and history of motion sickness of the patients in the two groups (p > 0.05). Most of patients (96.5% vs. 94.8% in groups A and B, respectively) had no history of chemotherapy (naive to chemotherapy) (p = 0.622). The most common types of cancers were osteosarcoma (30.3% vs. 21% in group A and B, respectively), acute lymphoblastic leukemia (21.5% vs. 24.6% in groups A and B, respectively), and Wilms tumor (19.6% vs. 22.8% in groups A and B, respectively). Doses and types of chemotherapy were similar in both groups, and there was no difference (data not shown).
Clinical and demographic findings of patients
| Characteristics | Group A (n = 56) | Group B (n = 57) | p-value |
|---|---|---|---|
| Gender, (%) | 0.644a | ||
| Female | 25 (44.6) | 23 (40.3) | |
| Male | 31 (55.4) | 34 (59.7) | |
| Age, years ± SD; | 10.21 ± 2.81 | 9.10 ± 3.13 | 0.689b |
| Min-Max | 7 - 18 | 5 - 16 | |
| Type of cancer; (%) | 0.891a | ||
| Acute lymphoblastic leukemia | 12 (21.5) | 14 (24.6) | |
| Rhabdomyosarcoma | 6 (10.8) | 7 (12.3) | |
| Wilms tumor | 11 (19.6) | 13 (22.8) | |
| Acute myeloid leukemia | 4 (7) | 3 (5.3) | |
| Osteosarcoma | 17 (30.3) | 12 (21) | |
| Hepatocellular carcinoma | 6 (10.8) | 8 (14) | |
| History of alcohol consumption, Yes (%) | 2 (3.5) | 3 (5.2) | 0.662a |
| Chemotherapy regimen, (%) | 0.974a | ||
| Dox + CP + Vincristine | 18 (32.1) | 20 (35.1) | |
| Dox + Cisplatin | 16 (28.6) | 15 (26.3) | |
| Dox + Dacarbazine + Vinblastine + Bleomycin | 13 (23.2) | 12 (21.1) | |
| Actinomycin D + Vincristine + CP | 9 (16.1) | 10 (17.5) | |
| Prior chemotherapy (non-naïve, %) | 2 (3.5) | 3 (5.2) | 0.622a |
| History of motion sickness, Yes (%) | 4 (7.1) | 6 (10.5) | 0.527a |
SD: Standard of deviation; NA: not applicable; BMI: body mass index; CP: Cyclophosphamide; Dox: Doxorubicin.
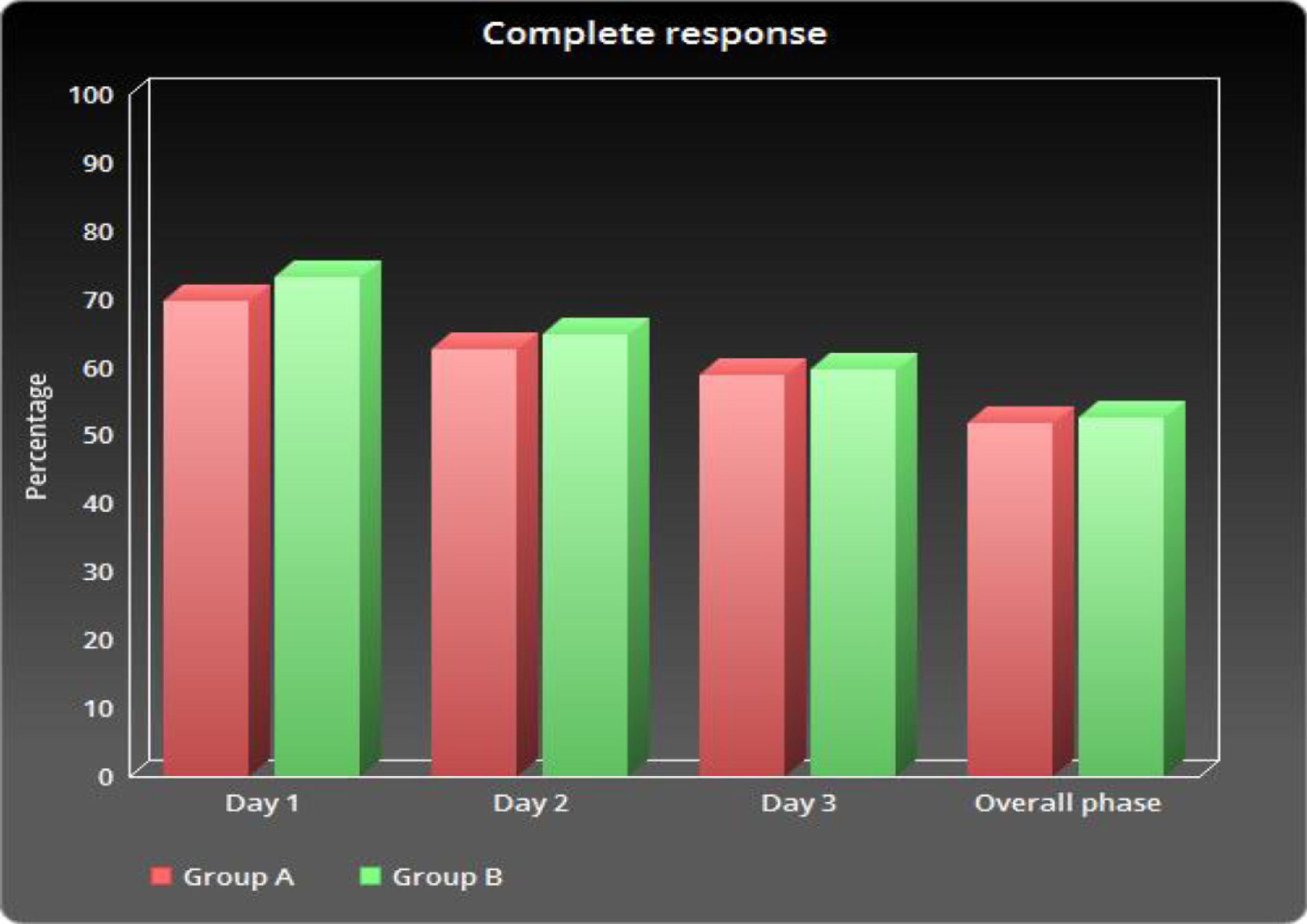
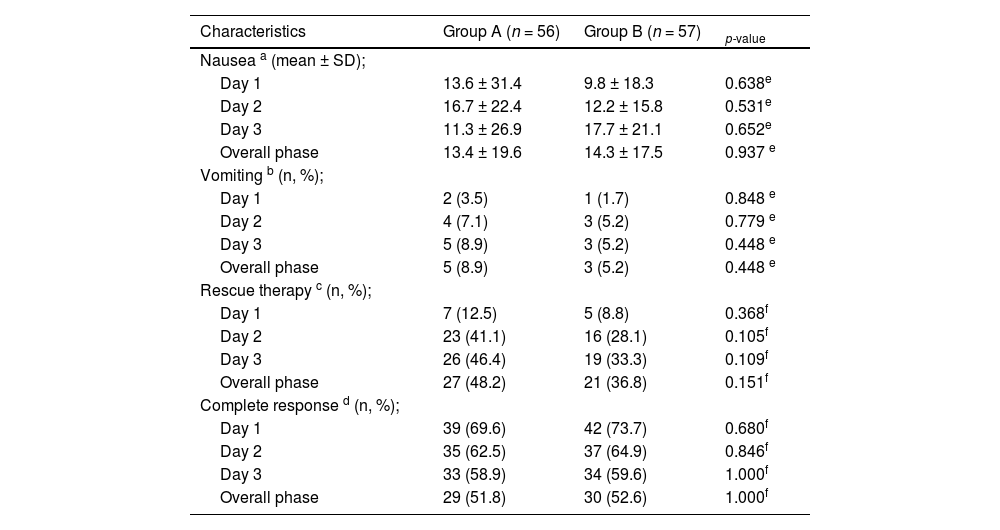
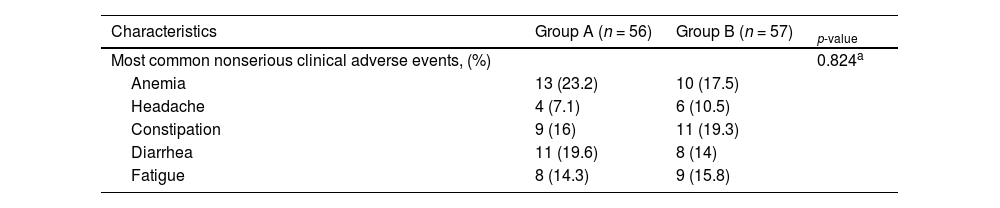
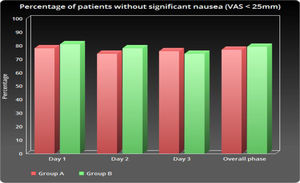
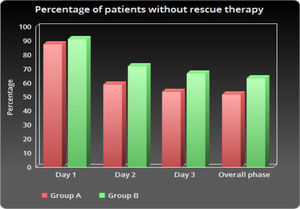
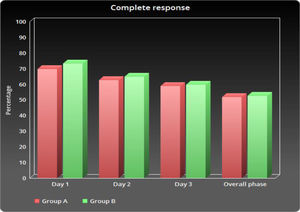
As shown in Table 2 and Figure 1, the number of patients without vomiting on the first day was similar in both groups (96.5% vs. 98.3%, respectively; p = 0.848). The severity of nausea on the first, second and third days, as well as significant nausea (VAS > 25mm) and overall nausea, were not significantly different between patients in the two groups (Table 2 and Figure 2). Moreover, the rate of use of breakthrough antiemetic medication in patients of both groups was almost the same (p > 0.05, Table 2 and Figure 3). As shown in Table 2 and Figure 4, the CR on the second and third days was almost the same in patients in both groups A and B (62.5% vs. 64.9% and 58.9% vs. 59.6%, respectively; p > 0.05). During the overall phase, the CR was observed in 51.8% of patients in group A and 52.6% of patients in group B (p = 1.000). No serious side effects were observed while taking the medication during the study (Table 3).
Status of CINV, rescue therapy and complete response of the patients in the two groups
| Characteristics | Group A (n = 56) | Group B (n = 57) | p-value |
|---|---|---|---|
| Nausea a (mean ± SD); | |||
| Day 1 | 13.6 ± 31.4 | 9.8 ± 18.3 | 0.638e |
| Day 2 | 16.7 ± 22.4 | 12.2 ± 15.8 | 0.531e |
| Day 3 | 11.3 ± 26.9 | 17.7 ± 21.1 | 0.652e |
| Overall phase | 13.4 ± 19.6 | 14.3 ± 17.5 | 0.937 e |
| Vomiting b (n, %); | |||
| Day 1 | 2 (3.5) | 1 (1.7) | 0.848 e |
| Day 2 | 4 (7.1) | 3 (5.2) | 0.779 e |
| Day 3 | 5 (8.9) | 3 (5.2) | 0.448 e |
| Overall phase | 5 (8.9) | 3 (5.2) | 0.448 e |
| Rescue therapy c (n, %); | |||
| Day 1 | 7 (12.5) | 5 (8.8) | 0.368f |
| Day 2 | 23 (41.1) | 16 (28.1) | 0.105f |
| Day 3 | 26 (46.4) | 19 (33.3) | 0.109f |
| Overall phase | 27 (48.2) | 21 (36.8) | 0.151f |
| Complete response d (n, %); | |||
| Day 1 | 39 (69.6) | 42 (73.7) | 0.680f |
| Day 2 | 35 (62.5) | 37 (64.9) | 0.846f |
| Day 3 | 33 (58.9) | 34 (59.6) | 1.000f |
| Overall phase | 29 (51.8) | 30 (52.6) | 1.000f |
CINV: Chemotherapy-induced Nausea and Vomiting; VAS: Visual analog scale; n: number.
Comparison of side effects between the two groups
| Characteristics | Group A (n = 56) | Group B (n = 57) | p-value |
|---|---|---|---|
| Most common nonserious clinical adverse events, (%) | 0.824a | ||
| Anemia | 13 (23.2) | 10 (17.5) | |
| Headache | 4 (7.1) | 6 (10.5) | |
| Constipation | 9 (16) | 11 (19.3) | |
| Diarrhea | 11 (19.6) | 8 (14) | |
| Fatigue | 8 (14.3) | 9 (15.8) |
N: number.
The current pilot study revealed that a single dose of aprepitant provided 3-day aprepitant-like efficacy in children who were receiving HEC. In this study, the occurrence of vomiting and nausea in group A was found in less than 10% and 20% of patients, respectively. In other words, the use of a single dose of aprepitant protects against vomiting in more than 90% of patients and nausea in more than 80% of patients. Previous studies have shown that adding aprepitant to standard treatment improves CR rates, compared to standard treatment in acute, overall and delayed phases.13,14 Hesketh et al. reported that the use of aprepitant in addition to the standard dual therapy, compared to the standard therapy, only improved the rate of CR in the acute (78% vs. 89%), delayed (55% vs. 75%) and overall phases (52% vs. 72%).15 Similar results were reported in our study Figure 5.
In accordance with our results, DiIorio et al. reported that a single dose of aprepitant reduced the number of episodes and severity of nausea and vomiting, the need for additional antiemetics and the length of stay.16 Furthermore, Saito et al. reported that aprepitant was safely used and may be equally useful for pediatric patients receiving HEC,17 which was consistent with the results of our study.
The CINV is one of the undesirable side effects of chemotherapy regimens for patients with cancer. Therefore, to improve the quality of life of patients receiving chemotherapy, it is necessary to assess the severity of the CINV. The effect of aprepitant-containing antiemetics in children receiving chemotherapy drugs in this study is similar to that in other studies.
Janelsins et al. reported that the application of serotonin receptor antagonists, along with DEX, can improve the CINV in the acute stage, but the delayed N/V has remained an important clinical issue.18,19 Aprepitant is one of the drugs approved by the FDA for the treatment of CINV in March 2003. Aprepitant is effective in preventing acute and chronic CINV in patients undergoing HEC, including cisplatin- and anthracycline-containing therapies.20
Herrington and colleagues reported a comparison trial with aprepitant, combined with palonosetron and DEX, in patients who were 18 years of age or older.21 Their Arm A patients received aprepitant 125 mg orally on day 1, followed by 80 mg orally on days 2 and 3, while Arm B received only aprepitant 125 mg orally on day 1 and placebo on days 2 and 3. All groups received palonosetron 0.25 mg (i.v.) on day 1, plus DEX on days 1 to 4. However, there were no statistical differences for emesis, nausea or the use of breakthrough antiemetic medication between Arm A and Arm B,21 which was consistent with our findings. In Arms A and B, 93% of the patients were vomiting-free from days 1 to 5,21 compared to 91.5% and 94.8%, respectively, in our study.
In another study, Oyama et al. evaluated the efficacy of aprepitant at 285 mg in patients with gastric cancer (median age 65 years) treated with S-1, plus cisplatin, for the prevention of CINV.22 They prescribed aprepitant at 125 mg orally 60 min before the cisplatin infusion, granisetron at 3 mg (i.v.) 30 min and DEX at 9.9 mg (i.v.) on day 1; oral aprepitant at 80 mg daily along with oral DEX at 8 mg on days 2 and 3, and; oral DEX at 8 mg on day 4. The CR was 98.1%, 88.7% and 88.7% in the acute, delayed and overall periods, respectively. The percentage of patients without vomiting during the first 24 hours was 98.1% and for the delayed and overall phases, 92.5% and 92.5%, respectively. In addition, the proportions of patients without significant nausea (VAS < 25) during the acute, delayed and overall periods were 98.1%, 69.8% and 69.8%, respectively. The proportions of patients without rescue therapy were 96.2%, 100% and 96.2 in the overall, acute and delayed periods, respectively.22
In a study, Grunberg et al. evaluated the efficacy of a single daily dose of aprepitant at 285 mg in patients with solid tumors for the prevention of acute and delayed CINV.23 They prescribed a single dose of aprepitant at 285 mg orally, along with palonosetron and DEX, to breast cancer patients with a median age of 52 years who were to undergo a chemotherapy regimen with cyclophosphamide and anthracycline. The CRs were 78%, 59%, and 50% in the acute, delayed and overall periods, respectively. The percentage of patients without vomiting during the first 24 hours was 100% and for the delayed and overall phases, 97% and 97, respectively. Additionally, the percentages of patients without significant nausea (VAS < 25) during the acute, delayed and overall periods were 75, 62% and 56%, respectively.23 However, it should be noted that the use of larger doses of aprepitant may interfere with chemotherapy drugs, such as cyclophosphamide and doxorubicin.
In a study on the efficacy of aprepitant and fosaprepitant in CINV in 26 children and teenagers with cancer (who were 11 months to 17 years), the authors found that the use of aprepitant and fosaprepitant had optimal effectiveness on decreasing CIVN, which was similar to the results of this study.24 In another study in patients aged 6 months to 17 years being treated with emetogenic chemotherapy, the authors recorded that the combination of aprepitant with ondansetron, with or without DEX, was useful in the prevention of CINV.25 These findings were, to some extent, similar to the results of this study and the limited difference could be attributed to the difference in the evaluated groups. In a study on the efficacy of aprepitant in the CINV of children suffering from cancer, the authors showed that aprepitant can be employed as a standard treatment for children suffering from severe CINV.26 Megan et al. showed that aprepitant appeared to be safe and well-tolerated in children weighing less than 40 kg, but that it might not completely reduce the CINV.27 In this study, it was shown that the combination of granisetron and aprepitant could render most patients CINV-free during days 1 to 3 after chemotherapy.
In a double-blinded randomized clinical trial, Kang et al. assessed the efficacy of aprepitant on the CINV of children suffering from cancer who were also undergoing chemotherapy. Their results revealed that the 3-dose oral aprepitant, in combination with ondansetron, with or without DEX, can significantly prevent the CINV in children undergoing chemotherapy, when compared with controls or those treated solely with ondansetron, with or without DEX.28 Shillingburg et al. conducted a study addressing the efficacy of aprepitant and fosaprepitant on the CINV among 26 children and teenagers with cancer. They reported no side effects due to the administration of aprepitant and, hence, they concluded that aprepitant and fosaprepitant could be well tolerated in children.29 No serious side effects were seen in this study.
ConclusionIn conclusion, the current study has shown that a single dose of aprepitant of 125 mg/kg was as effective as administering three doses of aprepitant over 3 days. Therefore, using a single dose of aprepitant of 125 mg/kg is less expensive and has similar efficacy for HEC regimens. Furthermore, the use of a single dose of aprepitant, in combination with other standard treatment regimens, to prevent the CINV in children who received highly emetogenic chemotherapy was safe and efficacious and could be beneficial.
We would like to thank the Research Council of Arak University of Medical Sciences, which has provided funding for this research (Grant 1719). We would also like to thank all the staff of the Blood and Oncology Department of the Amirkabir Hospital in Arak, Iran.