Erdheim-Chester disease (ECD) is a rare neoplasm from non-Langerhans cell (CD1a -, CD68+, S100-) histiocytes most commonly reported in elderly males. Patients with ECD have been reported to harbor several somatic mutations activating MAPK and PI3K pathways as BRAF+ (38–100%), BRAF wild-type, MAP2K1/K2, and PI3KCA mutations.1 Other MAPK mutations involving GTPases have also been described via RAS/RAF/MEK/ERK pathway in NRAS/ KRAS (Ras family) and ARAF (Raf family).2 These oncogenes are thought to promote early progenitor cells self-renew, increased foamy histiocyte infiltration, and chronic inflammation.
Given the ubiquitous presence of monocytic-originated cells, ECD may vary from one indolent spectrum to another with poor prognosis, representing a complex diagnosis as coexisting mixed Langerhans (CD1a + S100 + CD 207 +) and non-Langerhans (CD 1a – CD 68+ CD 163+) histiocytosis may develop. Most cases involve infiltration of long bones, retroperitoneum, orbits, skin, “hairy kidneys”, tests, and cardiovascular lesions. The central nervous system (CNS), gastrointestinal system (GIS), and skeletal involvement indicate a poor prognosis.3
Blood disorders have been reported before and after ECD onset, which are mostly myeloproliferative (MPS) disorders such as polycythemia vera, Essential Thrombocythemia, and myelofibrosis. Among MPS disorders, polycythemia vera (PV) is the most commonly reported with up to 20-year time lapse between PV diagnosis and ECD initial manifestation.4
The aim of this report is to describe a rare case of essential thrombocytosis (ET) who evolved, after 5 year-follow up, to ECD with extensive infiltration of long bones and to review the associations between MPS disorders and ECD.
Case reportA 72-year-old diabetic male showed deep venous thrombosis (DVT) in the right leg in 2015 that progressed to amputation. At the clinical investigation, thrombocytosis with a platelet count of 1.000.000/ µL was detected (normal leucocytes and hemoglobin). Bone marrow trephine showed megakaryocyte lineage with increased numbers of enlarged, mature megakaryocytes with hyperlobulated nuclei. JAK2V617F mutation was detected and no other criteria for chronic myeloid leukemia, PV, or Myelofibrosis was detected according to the 2016 WHO Classification of Tumors of Hematopoietic and Lymphoid tissues. The diagnosis of Essential Thrombocythemia was established, and treatment with hydroxyurea showed response with platelets counts between 300.000/ µL - 450.000/ µL in the last 5 years.
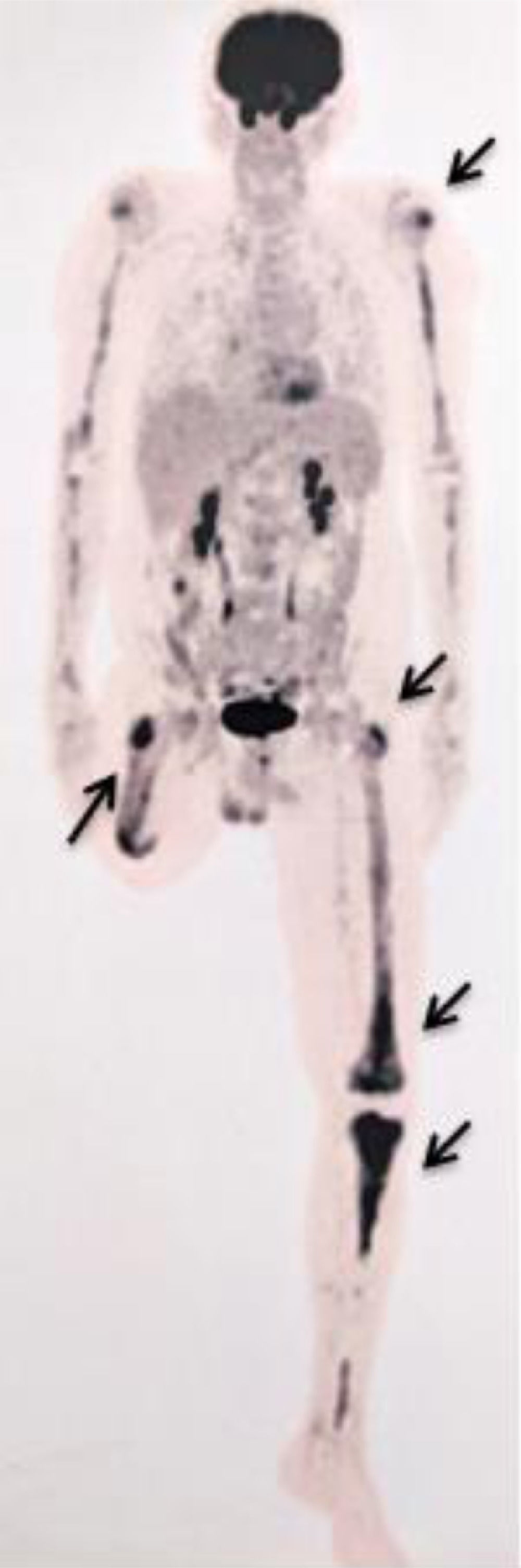
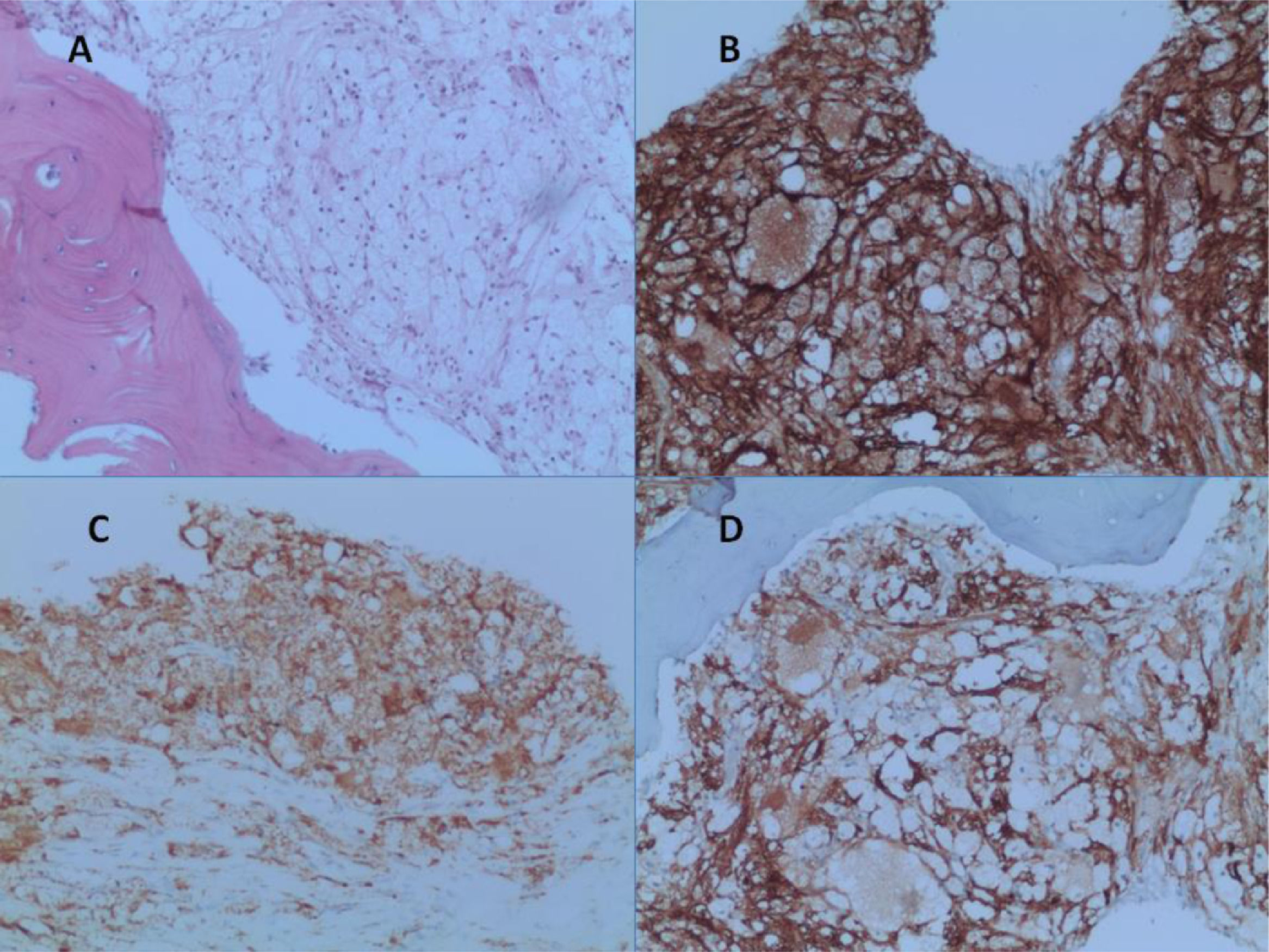
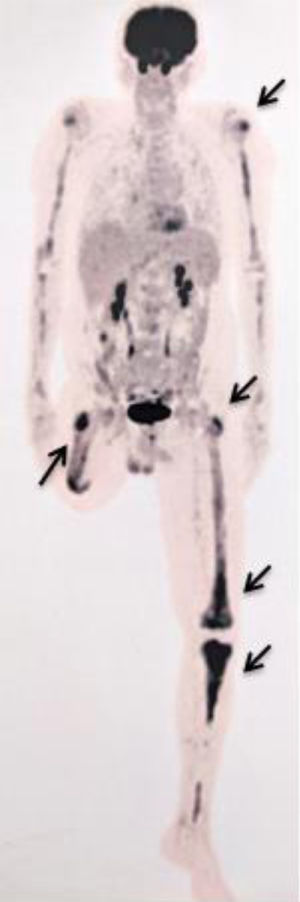
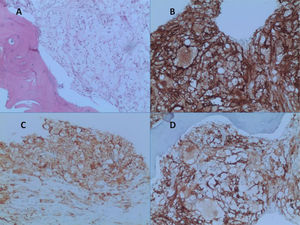
After a five-year follow-up, the patient reported very significant weight loss and bone pain. Peripheral blood test presented increased platelet count of 679.000/ µL and a PET-CT (due to bone pain) showed increased glycolytic metabolism and bone marrow density in the left frontal region (SUV 8.9); right humerus (SUV 3.6); left humerus (SUV 5.6); right femur (SUV 6.1); left femur (SUV 6.5); and left tibia (SUV 7.4) (Figure 1). Osteopenia in radius and femur was also observed. A paraffin-embedded bone marrow trephine of the right femur was performed and showed xanthomatous histiocytes positive for CD68, Factor XIII, and CD163 (Figure 2). The diagnosis of Erdheim-Chester disease was established according to clinical, histological and radiological criteria. BRAFV600E mutation was negative (By Sanger sequencing). The dosage of hydroxyurea was adjusted to maintain platelets count between 300.000/ µL - 450.000/ µL and the patient evolved into pulmonary cystic lesions in a new PET-SCAN with bizarre architecture and thickened walls suggestive of histiocytosis. The patient began treatment with Pegasys (peginterferon alpha-2a) and presents a stable clinical status at the moment.
DiscussionOur case here reported shows a rare association of ET and ECD. As Erdheim-Chester disease is a rare subtype of non-Langerhans cell histiocytosis (LCH)/CD68+ and ET is a myeloproliferative disorder, we initially thought this association was just coincidence, but after reviewing the most recent reports suggesting the origin of ECD cells from myeloid progenitor cells, made us believe there is a possible link between essential thrombocytosis and ECD, not a coincident event.
After reviewing the literature, we found reports demonstrating the association between ECD and myeloproliferative disorders in up to 10% of cases, most commonly chronic myelomonocytic leukemia (CMML).2 In a multi-institutional study of 170 ECD patients3 reported concomitant 19 myeloid neoplasms such as CMML (8 cases), ET (4 cases), MDS (myelodysplastic syndrome) (2 cases), Myelofibrosis (2 cases), AML (acute myeloid leukemia) (2 cases) and PV (1 case). Among these cases, many harbored kinase alterations characteristic of both ECD and myeloid neoplasms, but the most significant result was the detection of the same NRAS mutation in ECD lesions in the bone marrow and peripheral blood at CMML diagnosis.
We diagnosed ECD after PET-CT and biopsy from femur only after bone pain onset, very significant weight loss, and unexplainable increase of platelets. This uncommon association between myeloproliferative disorders and ECD brings low overall survival. Very recently, a study with 89 ECD patients1 indicated that the mean age at diagnosis was 55 years old (range: 34–80 yo). Of the entire cohort, 23 (25.8%) performed bone marrow biopsy during their disease most commonly due to abnormal peripheral blood count to rule out hematologic malignancy. The authors detected a concomitant/subsequent myeloid neoplasm in 3 of 89 (3.3%) ECD patients: 1) 75 yo, CMML, ECD 5 years after CMLL diagnosis (OS 3years); 2) 51 yo, Essential Thrombocytosis, ECD 17 years after ET diagnosis (OS 3.5 years); 3) 59 yo, CMML, ECD at the same year of CMML diagnosis (OS 3.5 years). Reinforcing the adverse clinical evolution, our case herein report developed lung disease after 1 year of ECD diagnosis, typically demonstrating disease progression in parallel to refractory ET with increased platelets. See Table 1 for all detected cases of ECD with MPS diagnosis regarding clinical features, cell markers, mutations, treatment, and outcomes.
Myeloproliferative disorders and Erdheim-Chester disease: case summary.
| Article | MPS/ Diagnosis | Clinical Features | Cell markers | Mutations/ Karyotype | Treatment after ECD diagnosis | Outcomes |
|---|---|---|---|---|---|---|
| Iurlo et al., 20165 | PV, ECD/ Clinical, Molecular | Splenomegaly, erythrocytosis, low EPO | CD 68 + Cd1a- S100 - | BRAFV600E, JAK2V617F, complex karyotype | IFN-alpha | Remission in 3 months |
| Tamura et al., 20186 | PV, ECD/ Clinical, Molecular | CNS mass, bone pain, erythrocytosis, low EPO | CD 68 + Cd1a- S100 - | BRAFV600E, JAK2V617F | Cladribine | Partial Remission |
| Baer et al., 19874 | PV, AML, hystiocytosis/ Clinical | Sternal mass | Alpha-1-antitrypsin, alpha-1 antichymotripsin | No report | Palliative radiotherapy | Death |
| Papo et al., 20173 | PV, ECD/ Clinical, molecular | No report | No report | BRAFV600E, JAK2, TET2, NRAS, U2AF1 | No report | No report |
| ET, ECD/ Clinical, molecular | No report | No report | BRAFV600E, JAK 2, TET2, MAP2K1,CALR | No report | No report | |
| AML, ECD/ Clinical, molecular | No report | CD 68 + Cd1a- S100 - | BRAFV600E, IDH2, TP53, TET2 | No report | No report | |
| Goyal et al., 20201 | ET, ECD/ Clinical, molecular | Pleural effusions, ascites, retroperitoneal soft-tissue infiltration | No report | BRAFV600E, JAK2V617F | Interferon-a, anakinra, and later trametinib | Death |
| Ghobadi et al., 20167 | AML, ECD/ Clinical, molecular | No report | CD34 +, CD68 + Cd1a - S100- | BRAFV600E, IDH2, R140Q | No report | No report |
| Sakr et al., 2018 | Burkitt, ECD/ Clinical molecular | No report | CD68 +, CD163 +, factor XIIIa +, BRAFV600E +CD1a -, CD21 -, CD23 - | BRAFV600E * | No report | No report |
| Goyal et al., 20207 | CMML, ECD/Clinical, molecular | Fatigue, weight loss, abdominal pain | No report | BRAFV600E | Anakinra, vemurafenib, dabrafenib | Loss of follow-up, death |
| CMML,ECD/ Clinical, molecular | Yellowish-red papules, bone pain | No report | BRAFV600E, ASXL1, KRAS | Hidroxyurea | Death | |
| Goyal et al., 20199 | CMML, ECD/ Clinical, molecular | Skin lesions, bone pain | CD 68 + Cd1a- S100 - | KRAS, ASXL1, CLDN1, THBS4, SYNC, ROBO2 | Hydroxycarbamide | Death |
| Papo et al., 20173 | CMML, ECD/ Clinical, molecular | No report | CD 68+, CD1a- | BRAFV600E, ASXL1 | No report | No report |
| CMML, ECD/ Clinical, molecular | No report | CD 68+, CD1a- | BRAFV600E,JAK2, IDH2 | No report | No report | |
| Bonnet et al., 20192 | CMML, ECD/ Clinical, molecular | Skin lesions | CD 68 + Cd1a- S100 - | KRAS, ASXL1, NRAS, DNMT3A |
PV: Polycythemia Vera; ECD: Erdheim-Chester Disease; AML: Acute Myeloid Leukemia; ET: Essential Thrombocythemia; EPO: Erythropoietin; CNS: Central Nervous System; CMML: Chronic Myelomonocytic Leukemia.
The monocyte cell subpopulations are diverse and involve both granulocyte-monocyte and monocyte-dendritic cells progenitors. Those progenitors have an overlapping differentiation potential and may suffer extrinsic signals induced differentiation, which hardens the tracking of driving mutations in histiocytosis. Also, BRAFV600E mutations were found in myeloid blood precursors of LCH and ECD patients.8 These could indicate early mutated genes in hematopoietic precursors of myeloproliferative neoplasms and characterize the ECD as a result of clonal progression. A recent study on tumor profiling of patients with non-Langerhans cell histiocytosis of 34 patients10 identified not only BRAFV600E and MAPK activating mutations but other BRAF mutations, gene fusions, gene amplification, and putative driver mutations. Those accumulated events indicate genomic instability and a pro-oncogenic microenvironment.
Nevertheless, the chronic inflammatory microenvironment and tissue-specific cell signaling may produce pathological subpopulations of monocytes and contribute to ECD pathogenesis. Interestingly, the M-CSF and lipid enriched serum treated monocytes differentiated into foam cells in vitro.8 Those could indicate a possible pathological insight into the formation of foam cells once osteoblasts are known as M-CSF producers and promoters of HSC (hematopoietic stem cells) maintenance and differentiation. Osteoblastic lesions are frequent in ECD and may play a significant role in disease pathogenesis.
This case highlights the clinical importance of evaluating adults with a myeloproliferative disorder for a concomitant histiocytosis. ECD concomitant to ET diagnosis is not a coincidence due to probable same cell origin.
Conflicts of interestThe authors declare no conflicts of interest.