Acute graft-versus-host disease (GVHD) is one of the major causes of morbidity and mortality in patients undergoing allogeneic hematopoietic stem cell transplantation (AHSCT) and has become the subject of several studies to understand and treat it.
ObjectiveThis study does a descriptive analysis of the apoptotic index (AI) evaluation and intestinal permeability (IP) alterations in association with the clinical, endoscopic and histopathological data on patients undergoing AHSCT, with emphasis on acute intestinal graft-versus-host disease (GVHD) diagnosis.
MethodsThirty-one patients were divided into two groups—one of patients with a clinical GVHD diagnosis and one of those without GVHD diagnosis.
ResultsThirteen deaths (41.9%) occurred during the study period, thereby reaffirming the severity of the alterations found in the patients. Fifteen patients subjected to 21 esophagogastroduodenoscopy procedures prior to D + 90 post-transplant had visible endoscopic alterations and 19 biopsies revealed histological alterations to the stomach and duodenum. Higher apoptotic indices, not reaching statistical significance, were observed in patients who died of graft versus host disease (GVHD), in the more acute forms of GVHD and where clinical GVHD was present. The intestinal permeability evaluation was performed on nine patients able to undergo it in the three proposed study periods, which showed alterations, some of which were pronounced even during pre-transplant and, therefore, the pre-conditioning phase.
ConclusionClinical judgment remains a fundamental tool in the diagnosis of GVHD. This study points to the known limitations of traditional diagnostic aids (endoscopy and histology) and points to new methods not usually employed in clinical practice.
The curative potential of allogeneic hematopoietic stem-cell transplantation (AHSCT) is based on lethal doses of chemotherapy and/or radiotherapy utilized in conditioning regimes and on the graft-versus-leukemia (GVL) effect. However, the GVL effect mediated by the allogeneic graft is typically observed along with graft-versus-host disease (GVHD).1,2
Acute GVHD, particularly of the gastrointestinal tract, is characteristically a major complication in AHSCT. It is difficult to handle, as it is associated with malnutrition, injury to organs, as well as with frequent and generally acute infections triggered not only by GVHD itself, but also by immunosuppressive therapy.3,4
It is difficult to diagnose acute GVHD by applying clinical parameters alone, as signs and symptoms are nonspecific and because there are several other conditions that may mimic or coexist with acute GVHD. In the case of acute GVHD of the GI tract, diagnostic confirmation may require endoscopic procedures with biopsies.5,6
Improvements in grafting and GVHD prophylaxis reduced its incidence and severity and, as a result, pathologists are more prone to notice minimal alterations instead of histologically advanced alterations, which may lead to diagnostic failures.7,8 Moreover, endoscopic examinations frequently give rise to complications such as hemorrhage due to thrombocytopenia. As such, development of non-invasive methods to diagnose gastrointestinal GVHD can contribute to improve diagnosis, enabling treatment to be started earlier with less risk to the patient.
Intestinal mucosa permeability is impaired in patients undergoing AHSCT, according to several studies utilizing intestinal permeability tests to evaluate mucositis.9–11 However, there are few studies in the literature utilizing such tests to diagnose GVHD.12,13 The gold standard for diagnosing acute GVHD of the GI tract is the histopathology study of samples from gastric, duodenal or colon biopsies obtained from endoscopy.
Considering the need for alternatives for GVHD diagnostic studies, we made a descriptive analysis of diagnostic methods, including upper gastrointestinal endoscopy, conventional histopathological studies, intestinal permeability evaluation and the apoptotic index evaluation, a quantitative measure of apoptosis, with emphasis on the diagnosis of acute intestinal GVHD.
Patients and methodsPatientsThirty-one consecutive patients undergoing AHSCT were evaluated between August 2007 and May 2010. Patients agreed to take part in the study after receiving the necessary explanations and signing an informed consent. The patients were admitted, evaluated and followed under the routines of the Transplantation Unit.
A standard protocol was followed by all the patients for clinical evaluation and performance of complementary tests, according to the following steps:
- 1
On admission to the Transplant Unit, forms were filled out with socio-demographic, clinical and laboratory data. An intestinal permeability study was performed preferentially before or during the conditioning regime.
- 2
Patients with no symptoms of GI tract impairment: clinical evaluation, laboratory data and intestinal permeability studies at or around D + 14 and D + 30 post-transplant.
- 3
Patients with GI tract impairment symptoms (nauseas/vomiting, pronounced hyporexia and diarrhea) at any moment between D + 14 and D + 90 post-transplant: clinical GVHD evaluation and classification, clinical evaluation, laboratory data, performance of an upper digestive endoscopy with stomach and duodenum biopsies. The biopsy samples were sent to the Pathology Laboratory of the Hospital.
All patients included in the study were clinically monitored from admission until hospital discharge or D + 90 post-transplantation, whichever came first, and clinical diagnosis of GVHD was performed according to the criteria defined by Ferrara,14 starting on D + 14 post-transplantation. Criteria used for staging acute clinical GVHD were those revised by the Consensus Conference on Acute GVHD Grading.15 Patients were separated into two groups: those with mild to moderate acute clinical GVHD (final staging grades I or II) and those with severe acute clinical GVHD (final staging grades III or IV).
Intestinal permeability testsThe intestinal permeability tests were performed in accordance with a standardized technique,16 using a meal of a standard solution containing 6.25 g of 95% PA crystalline lactulose, Sigma–Aldrich® and 3.0 g of crystalline mannitol PA, Sigma–Aldrich®, after a 6 h fast and followed by a 5 h urine collection. A 50 ml aliquot of urine was separated off for analysis of urinary lactulose and mannitol concentrations by High Performance Liquid Chromatography (HPLC), followed by the calculation of their excretion rates (in relation to the amount of sugar ingested). Finally, the ratio between the urine excretion rates of the two substances was obtained (lactulose/mannitol excretion rates).
Upper digestive endoscopy and histologyUpper digestive endoscopy examinations were performed according to service routines. Biopsies were performed only when the platelet count was equal to or above 50,000 mm³. Patients with lower counts received transfusion of platelet concentrates prior to the procedure.
The samples of gastric and duodenal tissue stored in 10% formalin were sent to the Pathology Laboratory to be processed in paraffin and stained with Hematoxylin-eosin (HE); the stomach biopsy samples were also stained for Helicobacter pylori with Giemsa.
All biopsies were evaluated by two experienced pathologists specialized in the upper GI tract, with research on apoptosis, using Sidney’s modified histological classification.17 Alterations compatible with intestinal GVHD were evaluated according to the NIH Consensus5 and described separately. Edema was evaluated according to intensity: none, moderate or severe.
Obtaining the apoptotic index (AI) and immunohistochemistryThe Apoptotic Index (AI) was obtained in a blind fashion on terminal uridine nick-end labeling (TUNEL)-stained laminae.18 Apoptotic cell quantification was made by a single observer. As an inclusion criterion, the simultaneous presence of at least three of the typical morphological characteristics was adopted: anoikia—cell retraction and loss of adhesion between cells and basal membrane; cytoplasmic and nuclear condensation—compaction of the nuclear chromatin into dense uniform masses, aligned on the inner side of the nuclear membrane, sometimes forming crescent images; nuclear fragmentation—convolution and fragmentation of the nuclear membrane—without karyorrhexis or rupture, and; cellular fragmentation—with formation of the apoptotic bodies.
The AI was determined by the following formula: (AI) = (Σ no. of apoptotic cells/Σ no. of total cells) ×100.
To validate the morphological criteria in order to quantify the AI and the TUNEL technique18 (Apoptag® Peroxidase in situ, Apoptosis Detection Kit, Cat S7100, Chemicon), immunohistochemistry was also performed for Bax (Mouse x Bax, Invitogen, Cat.#18-0218).
For the morphometrics study, digital images of the histological lamina were acquired utilizing the video-camera system, coupled to the CH30 Olympus light microscope with a 40× objective to make the apoptosis cell count and determine the AI. The images were transferred by the video-camera system to a computer and captured through Honestech TVR2.5 Software. The acquisition of images was performed at the Morphometrics Laboratory of the General Pathology Department at the University. The IA Quantification was made using the Media Cybernetics Image Pro-Plus, version 4.0, image analyzer.
Statistical analysisThe collected data were stored in a data bank created in the EpiData 3.1 software. The data for the intestinal permeability study were analyzed utilizing the SSPS version 13.0 software and the apoptosis index data were analyzed utilizing the Graph Pad Prisma version 5.0 software.
Ethical aspectsThe study was approved by the Research Ethics Committee (UFMG, ETIC 092/07).
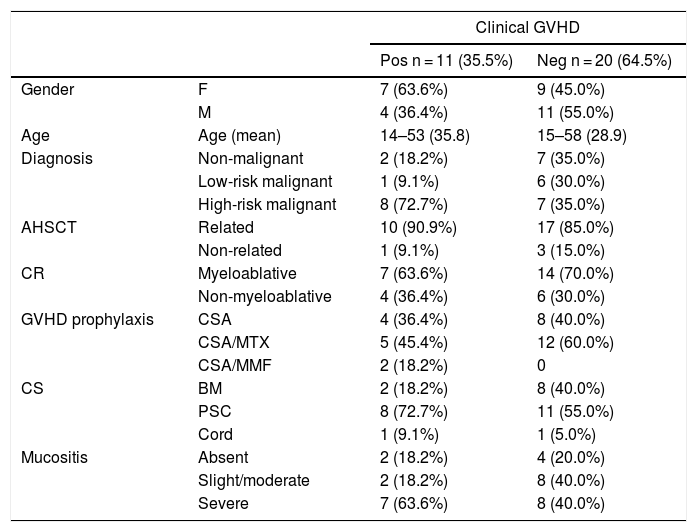
ResultsPatientsThirty-one patients admitted for AHSCT were included in the study. There were no statistical differences of sex, age, diagnosis, type of transplantation, conditioning regime, cell source and GVHD prophylaxis in the two groups analyzed, as Table 1 shows.
Demographic characteristics, diagnosis and treatment.
| Clinical GVHD | |||
|---|---|---|---|
| Pos n = 11 (35.5%) | Neg n = 20 (64.5%) | ||
| Gender | F | 7 (63.6%) | 9 (45.0%) |
| M | 4 (36.4%) | 11 (55.0%) | |
| Age | Age (mean) | 14–53 (35.8) | 15–58 (28.9) |
| Diagnosis | Non-malignant | 2 (18.2%) | 7 (35.0%) |
| Low-risk malignant | 1 (9.1%) | 6 (30.0%) | |
| High-risk malignant | 8 (72.7%) | 7 (35.0%) | |
| AHSCT | Related | 10 (90.9%) | 17 (85.0%) |
| Non-related | 1 (9.1%) | 3 (15.0%) | |
| CR | Myeloablative | 7 (63.6%) | 14 (70.0%) |
| Non-myeloablative | 4 (36.4%) | 6 (30.0%) | |
| GVHD prophylaxis | CSA | 4 (36.4%) | 8 (40.0%) |
| CSA/MTX | 5 (45.4%) | 12 (60.0%) | |
| CSA/MMF | 2 (18.2%) | 0 | |
| CS | BM | 2 (18.2%) | 8 (40.0%) |
| PSC | 8 (72.7%) | 11 (55.0%) | |
| Cord | 1 (9.1%) | 1 (5.0%) | |
| Mucositis | Absent | 2 (18.2%) | 4 (20.0%) |
| Slight/moderate | 2 (18.2%) | 8 (40.0%) | |
| Severe | 7 (63.6%) | 8 (40.0%) | |
GVHD = graft-versus-host disease; pos = positive; neg = negative; AHSCT = allogeneic hematopoietic stem-cell transplantation; CR = conditioning regimen; CSA = cyclosporine A; MTX = methotrexate; MMF = mophetylmicophenolate; CS = cell source; BM = bone marrow; PSC = peripheral stem cells.
Eleven patients were diagnosed with acute GVHD on clinical grounds (35.5%), while the remaining 20 patients (64.5%) were considered free of GVHD. All 11 patients with acute GVHD were considered to have gastrointestinal GVHD. Three of them also had acute skin and liver GVDH, two had skin GVDH and one had liver GVDH.
Thirteen of the 31 patients (41.9%) died during the study period. Deaths occurred between D + 8 and D + 66 post-transplantation with a 37-day median. Four of the deaths (36.4%) were in Group 1 (n = 11), three were from acute grade IV GI tract GVHD and one from infection, the latter with a severe acute GVHD diagnosis too. Nine deaths (45.0%) occurred in group 2 (n = 20), four of them from sinusoidal obstruction syndrome, four from respiratory insufficiency and one from infection/sepsis.
Endoscopic alterationsFifteen patients with GI tract symptoms underwent upper digestive endoscopy in the period up to D + 90 post-transplantation. In addition to the suspicion of GVHD, endoscopy was performed to dismiss esophageal candida, cytomegalovirus (CMV) infection according to clinical and/or serological evidence, as well as other gastroduodenal pathologies. Five of the patients underwent more than one endoscopy. There were visible endoscopic alterations in 11 of the 21 (52.4%) procedures performed. The most commonly found mucosa abnormalities were esophageal erosions and ulcers, moderately intense enanthematous gastritis, subepithelial hemorrhage areas in the body, antral erosions and pronounced pangastritis with micronodularity, erosive duodenitis, enanthematous duodenitis, serrated appearance of the mucosa and duodenal petechiae. Esophageal moniliasis was also found in the histology sample of one patient.
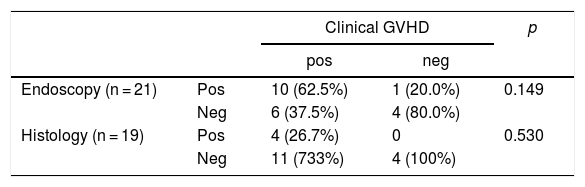
Matching was positive between clinical suspicion of GVHD at the time of the examination and the suggestive endoscopic observations (Table 2) in 10 of the 21 (47.6%), and negative in six of the 21 (28.6%) procedures performed, with no statistical significance.
Match between the acute GVHD clinical diagnosis and the endoscopic and histological post-AHSCT alterations.
| Clinical GVHD | p | |||
|---|---|---|---|---|
| pos | neg | |||
| Endoscopy (n = 21) | Pos | 10 (62.5%) | 1 (20.0%) | 0.149 |
| Neg | 6 (37.5%) | 4 (80.0%) | ||
| Histology (n = 19) | Pos | 4 (26.7%) | 0 | 0.530 |
| Neg | 11 (733%) | 4 (100%) | ||
GVHD = graft versus host disease; AHSCT = allogenic hematopoietic stem cell transplantation; pos = positive; neg = negative.
Biopsies were not performed in two procedures due to evident signs of bleeding. Esophagus biopsies were only performed when viral or fungal infection was suspected.
The histological alterations suggestive of GVHD found in the stomach (n = 3), were a predominantly leukocyte inflammatory infiltration, necrosis of the glandular epithelium, increased apoptosis and presence of apoptotic corpuscles. In the duodenum (n = 3) atrophy and ulceration of the mucosa, flattened villi with a decreased villous to crypt ratio, cell vacuolization, eroded areas, regenerative atypias in the crypts and frequent nuclear alterations with increased apoptosis, as well as a granulomononuclear inflammatory infiltrate, congestion and areas of hemorrhage. Stomach and duodenum GVDH was found simultaneously in two patients.
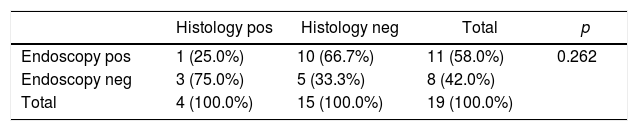
Endoscopic alterations suggestive of GVHD only matched the histological confirmation in one of the 19 endoscopies, as shown in Table 3. Only four of the 19 biopsy samples (21%) had a histological GVHD diagnosis, also suspected on clinical grounds. Signs of mild unspecific chronic gastritis or active chronic duodenitis were seen in five patients with clinical suspicion of GVHD, none of whose etiology was determined by histology.
Other histological alterations other than signs suggestive of GVHD were esophageal moniliasis (n = 1), Helicobacter pylori in the stomach (n = 1) and inclusions suggestive of cytomegalovirus infection in stomach (n = 2) and duodenum (n = 1) biopsies.
Apoptotic indexThe apoptotic index (AI) analysis and immunohistochemistry studies were performed on only nine patients because the biopsy samples did not display a sufficient number of fields for statistical analysis, chiefly due to the presence of artifacts and fragmentation. The AI was calculated in 10 fields for each patient. AI values ranged from 3.791% to 24.51%. The mean AI of the 10 fields analyzed for each patient ranged from 6.503% to 11.92% and the median from 6.198% to 12.43%.
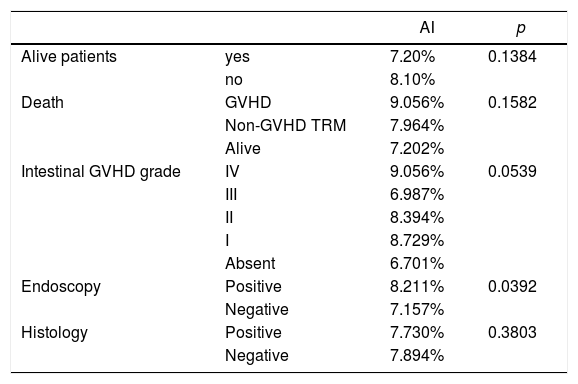
The median of the AI values and its relation to deaths from GVHD or TRM (transplant-related mortality), GVHD staging, endoscopic diagnosis and histological diagnosis (Table 4) were evaluated. Higher AIs were observed in the worst outcomes, without statistical significance. In relation to the severity of GVHD, the difference among AIs was close to statistical significance. A higher AI was significantly correlated with endoscopy classified as suggestive of GVHD.
Apoptotic Index values relating to deaths, deaths from GVHD or from TRM, staging of GVHD, endoscopic diagnosis and histological diagnosis.
| AI | p | ||
|---|---|---|---|
| Alive patients | yes | 7.20% | 0.1384 |
| no | 8.10% | ||
| Death | GVHD | 9.056% | 0.1582 |
| Non-GVHD TRM | 7.964% | ||
| Alive | 7.202% | ||
| Intestinal GVHD grade | IV | 9.056% | 0.0539 |
| III | 6.987% | ||
| II | 8.394% | ||
| I | 8.729% | ||
| Absent | 6.701% | ||
| Endoscopy | Positive | 8.211% | 0.0392 |
| Negative | 7.157% | ||
| Histology | Positive | 7.730% | 0.3803 |
| Negative | 7.894% |
AI = Apoptotic index; GVHD = Graft-versus-host disease; TRM = transplantation-related mortality.
Of the 31 patients included in the study, 25 (80.64%) underwent intestinal permeability evaluation in the pre-transplant phase, 21 (67.74%) around D + 14 post-transplantation and 11 (35.48%) around D + 30 post-transplantation, while only nine (29.0%) were able to perform the examination in the three periods, as initially scheduled.
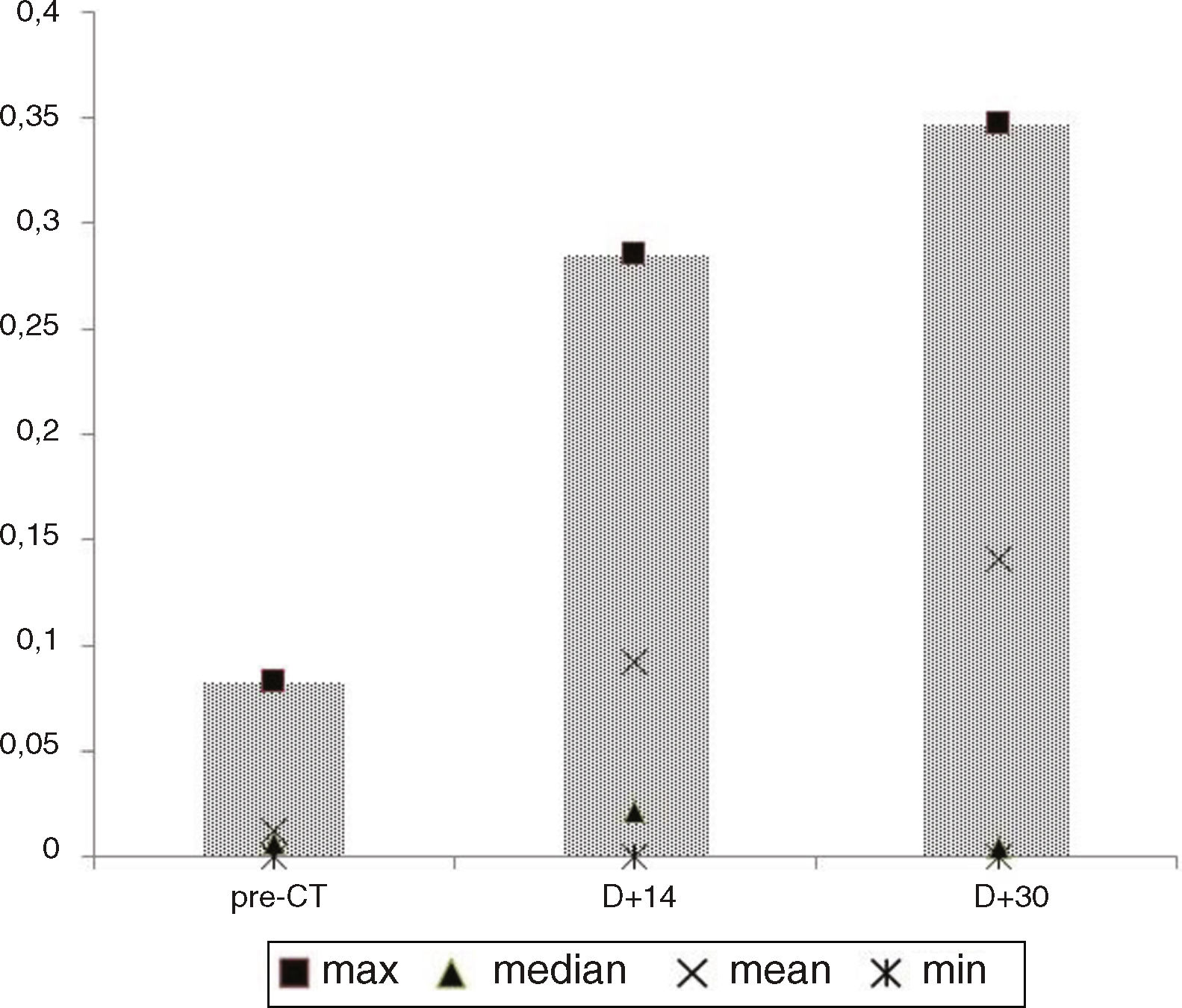
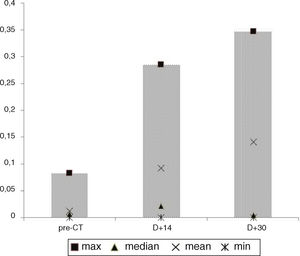
Paired Student’s t-tests comparing the different intestinal permeability collection times did not reveal statistical significance. The several losses between study phases may have impaired analysis. The graph in Fig. 1 shows a considerable increase in the separate lactulose/mannitol excretion rates (L/M R) means (non-paired), rising from 0.0127 in the pre-transplant phase to 0.092 and 0.141 on days +14 and +30, respectively. However, these were not accompanied by similar alterations in the median, which showed a rise of 0.007 to 0.022 between the pre-transplant phase and D + 14, dropping to 0.005 on D + 30. Two cases outside the curve, with a L/M R of 0.860 on D + 14 and of 1.142 on D + 30, not represented in Fig. 1 to avoid affecting the graph’s scale, helped increase the separate means on those dates.
Twenty four percent of the cases in the pre-transplant period, 60% on D + 14 and 27% on D + 30 had an R L/M value higher than 0.012, corresponding to the mean +2 standard deviations of a control sample of 16 healthy people of both sexes, previously evaluated at the intestinal permeability laboratory. There was no relation between the R L/M values observed and clinical outcomes, such as the development of clinical GVHD and death.
DiscussionThe difficulties for the establishment of tests that aid in the diagnosis of GVHD are evident in this study.
The macroscopic aspect of the upper digestive tract mucosa presented a reasonable correlation with the clinical diagnosis. However, their correlation with the histological diagnosis was low, indicating that both tests, although complementary, add little to the clinical suspicion, especially when negative. Positive tests could have a more significant contribution, considering the higher specificity of both the endoscopic aspect and the biopsy, but attention should be drawn to the fact that in only 1 case a coincidence between biopsy and positive endoscopic appearance was found. It should be noted that the endoscopies were performed by two experienced endoscopists who work together and perform all the endoscopies of the bone marrow-transplanted patients. The rate of false-negative histology in upper-gut GVHD is significant; what the endoscopist sees as mucosal edema, erythema, and friability are often invisible to the pathologist.19
The histological diagnosis for acute GVHD, regarded as the gold standard, is accomplished if the biopsy sample shows an array of characteristic findings.6,20–23 The importance of biopsy has been emphasized in the literature by studies showing that 30–80% of patients subjected to AHSCT with persistent clinically unexplained GI tract symptoms present histological GVHD, despite having a mucosa of normal appearance under endoscopy.6,20
Another point worth mentioning is that the endoscopic collection of biopsies is not always possible. It frequently results in an unsuitable or unrepresentative sample, notwithstanding the occurrence of complications. In the study, it was possible to perform biopsies on 19 of the 21 endoscopic procedures, but only nine of those samples had enough technical quality to enable the apoptotic index and the immunohistochemistry studies to be performed.
Concerning the AI, the differences found did not allow for conclusions, but could point to its potential use. In spite of a lack of statistical significance, the higher apoptotic indices observed in patients who died from GVHD, in the more severe forms of GVHD and in the mere presence of GVHD, could underlie the possible relevance of this methodology in studies with greater statistical power.
As regards apoptosis, a distinction must be drawn between its role as a physiological mechanism in the renovation of the GI tract epithelium and as representing resolution of inflammatory processes. On examining literature not related to AHSCT on apoptosis of digestive cells, it is observed that the GI tract mucosa presents high mitosis and apoptosis rates as a tissue homeostasis mechanism. Apoptosis represents an important regulator of the number of cells and minimizes susceptibility to malignant transformation.24
In inflammation, aggression on cells induces necrosis with the recruitment of leukocytes. When the leukocytes in the inflammatory focus manage to eliminate the aggressive agent and engulf the necrotic cell debris, a decrease in production and activation of the inflammation mediators will reduce the recruitment of new leukocytes and trigger apoptosis of the leukocytes present in the inflammatory focus. Thus, apoptosis may be a resolution marker of the inflammatory process.25–28
Improvements in the quality of post-transplant engraftment and prophylaxis significantly reduced the incidence and severity of acute GVHD. As a result, pathologists are more prone to value milder alterations and, indeed, a single apoptotic cell in a biopsy can be present. Nguyen et al.7 showed that samples with mild findings containing a single apoptotic cell represented 11% of the cases and can be a diagnostic problem for the pathologist. Socié et al.23 utilized the TUNEL18 method to evaluate apoptosis under an electronic microscope and in the 95 patients studied, the univariated and multivariated TRM analyses on D + 90 and within a year post-transplantation, in comparison with histological findings and clinical data, showed that finding over five cells per cellular field is significantly associated with a bad prognosis.
Evaluation of apoptosis by the apoptotic index method18 differs from the field count method employed in other studies, which prevents comparison between them. Ideally, the AI should be referred to a control group of healthy patients, not carriers of gastroduodenal pathologies, which we were not able to perform. Nevertheless, differences between patients with or without clinical GVHD should be of value, since it is a quantitative method for evaluating apoptosis.
It is worth mentioning the statistical significance in the correlation between the apoptotic index and the macroscopic endoscopic findings, with a positive predictive value of 91%, even though the low number of AI observations does not allow for definitive conclusions.
The role of the intestinal mucosal barrier injury (MBI) in the development of GVHD has been emphasized,9–11 indicating the MBI as a trigger for the appearance of acute GVHD, from both the liberation of cytokines and translocation of bacterial toxins.9
Non-invasive tests, such as sugar permeability tests, can detect alterations in permeability due both to functional alterations in the epithelium and to the loss of epithelial surface.
We found several difficulties in performing the IP tests, as already described.29 The first of them was the compliance by patients who found it difficult to drink the sugar solution during the conditioning, due to the associated nauseas and vomiting, and during the post-transplant mucositis phase, due to the fear of worsening nausea, vomiting, abdominal pain or diarrhea.
Another difficulty was the clinical severity of some patients who, even with the use of nasogastric or nasoenteric tubes to infuse the sugars, had symptoms of sepsis and severe renal or respiratory insufficiency, which contraindicated the performance of these examinations.
Therefore, the number of examinations decreased progressively as the post-transplant phase advanced, not allowing a sequential evaluation of the MBI injury with an appropriate sample.
The considerable increase in the range of L/M R values from the pre-transplant phase to D + 14, observable in the graph in Fig. 1, as well as the existence of the parametric distribution in healthy patients, which is modified to a non-parametric distribution in leukemic patients, particularly in the mucositis period, had already been observed previously by our group.11 It may be supposed that the intensity of the injury related to the mucositis and the cytokine storm so affect the mucosal barrier that the normal permeation characteristics become completely affected. It would be reasonable to suppose that patients with greater solute permeation indices would show a greater incidence of GVHD, which we were not able to show. A similar pattern of sequential permeability tests in ASCTH has been observed,30 with detection of lactulose/rhamnose ratio values of 0.04 in the pre-conditioning period, with a standard deviation of 0.02, which rose by up to 4 to 5 times, on average, with standard deviations of up to 0.14 between days D + 7 and D + 21.
It is still to be determined if this wild variation of the intestinal permeability tests from conditioning to mucositis phases is related, to any extent, to the development of GVHD or if it is just a manifestation of the significant mucosal damage following conditioning or even a consequence of a previous damage to the mucosa related to chemotherapies performed before transplantation. It also remains to be determined if there is any role for permeability tests as a non-invasive diagnostic tool for acute GVHD.
This study points to the known limitations of traditional diagnostic aids (endoscopy and histology). Regarding the methods not usually employed in clinical practice (AI and IP), this preliminary study suggests that additional studies on the AI, with larger samples and special attention to sample retrieving, may show its potential as a quantitative method. As regards to IP tests, in spite of its importance as a pathophysiological explanation and experimental tool, important limitations were disclosed related to compliance by patients and large confidence intervals. Additionally, the training of pathologists and endoscopists, adequate preparation of samples and close discussion between both and the transplantation team seem important to the clinical and research approach to the diagnosis of GVHD. To further aid in the clinical judgment, which remains a fundamental tool in the diagnosis of GVHD, the development of new methods must be pursued.
Conflicts of interestThe authors declare no conflicts of interest.
The Research Project obtained a grant from the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG).
Study performed at the Transplant Unit of the Hospital das Clinicas - Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil.