We performed cost-effectiveness and cost-utility analyses of the modified International Consortium on Acute Promyelocytic Leukemia protocol in Mexico for the treatment of acute promyelocytic leukemia Acute Promyelocytic Leukemia.
MethodsWe performed a three-state Markov analysis: stable disease (first line complete response [CR]), disease event (relapse, second line response and CR) and death. The modified IC-APL protocol is composed of three phases: induction, consolidation and maintenance. Cost and outcomes were used to calculate incremental cost-effectiveness ratios (ICERs); quality-adjusted life-years were used to calculate incremental cost-utility ratios (ICURs).
ResultsThe CR was achieved in 18 patients (90%), treated with the IC-APL protocol as the first-line option; one patient (5%) died in induction, another one never achieved CR (5%); of the 18 patients that achieved CR, 1 relapsed (5.5%). The median treatment cost of the IC-APL protocol was $21,523 USD. The average life-year in our study was 7.8 years, while the average quality-adjusted life-year (QALY) was 6.1 years. When comparing the ICER between the IC-APL and the all-trans retinoic acid (ATRA) plus arsenic trioxide (ATO) protocols, we found the different costs of $6497, $19,133 and $17,123 USD in Italy, the USA and Canada, respectively. In relation to the ICUR, we found the different costs to be $13,955 and $11,979 USD in the USA and Canada, respectively.
ConclusionTaking into account the similar response rates, lower cost and easy access to the modified IC-APL regimen, we consider it a cost-effective and cost-utility protocol, deeming it the treatment of choice for our population.
Acute promyelocytic leukemia (APL) is distinguished by the presence of a reciprocal translocation between chromosomes 15 and 17, or t(15;17).1 Depending on the country, APL usually accounts for between 5 and 12% of all acute myeloid leukemia (AML) cases,2 and is usually associated with disseminated intravascular coagulation (DIC), hemorrhagic syndrome and central nervous system involvement, along with typically acute leukemia complications, such as infections.3
From the discovery of the promyelocytic leukemia retinoic acid receptor (PML-RAR) alpha fusion protein, APL treatment was revolutionized by the introduction of the all-trans retinoic acid (ATRA),4,5 a vitamin A derivative that induces differentiation of immature promyeloblasts into mature granulocytes.6 Its combination with arsenic trioxide (ATO) is considered the first-line treatment of APL, able to induce a complete remission (CR) in ≥ 90% of patients, with a 2-year overall survival (OS) of 99%.7 More recently, ATO has been approved by the regulating organizations in both the USA and Europe for the treatment of newly diagnosed patients with low-to-intermediate risk APL.8
APL is associated with a high risk of early hemorrhagic death during induction, ranging from 3 to 11% in some studies.9,10 In developing countries, the mortality rate at induction was significantly higher (up to 32%).11 In order to narrow this gap, the International Consortium on Acute Promyelocytic Leukemia (IC-APL) developed a protocol with standardized supportive care and accessible drugs, which helped diminish the early mortality rate and improve the OS in developing countries, indirectly reducing the economic burden associated with the treatment of APL.3,12 Such a regimen is comprised of three phases: an induction phase (ATRA plus daunorubicin [DNR]), a consolidation phase (ATRA plus DNR, cytarabine [Ara-C] and mitoxantrone [MTZ]) and a maintenance phase (ATRA plus methotrexate [MTX] and 6-mercaptopurine [6-MP]).3,12
The IC-APL protocol has provided excellent clinical results in the treatment of APL, with a 2-year OS in adults, initially achieving only a 50% OS, with an induction mortality rate reaching 30%11 and increasing the OS to 80% and reducing mortality by half, after undergoing some modifications.3 Additionally, arsenic trioxide (ATO) has shown adequate responses with low toxicities in developing countries,13 however, it is not reimbursed by the public health system in Mexico and its cost is considerably high.
The objective of this study was to perform a cost-effectiveness analysis (CEA) and a cost-utility analysis (CUA) of the modified IC-APL protocol in Mexico, comparing it to the costs of the first-line treatment ATRA plus ATO in other countries.
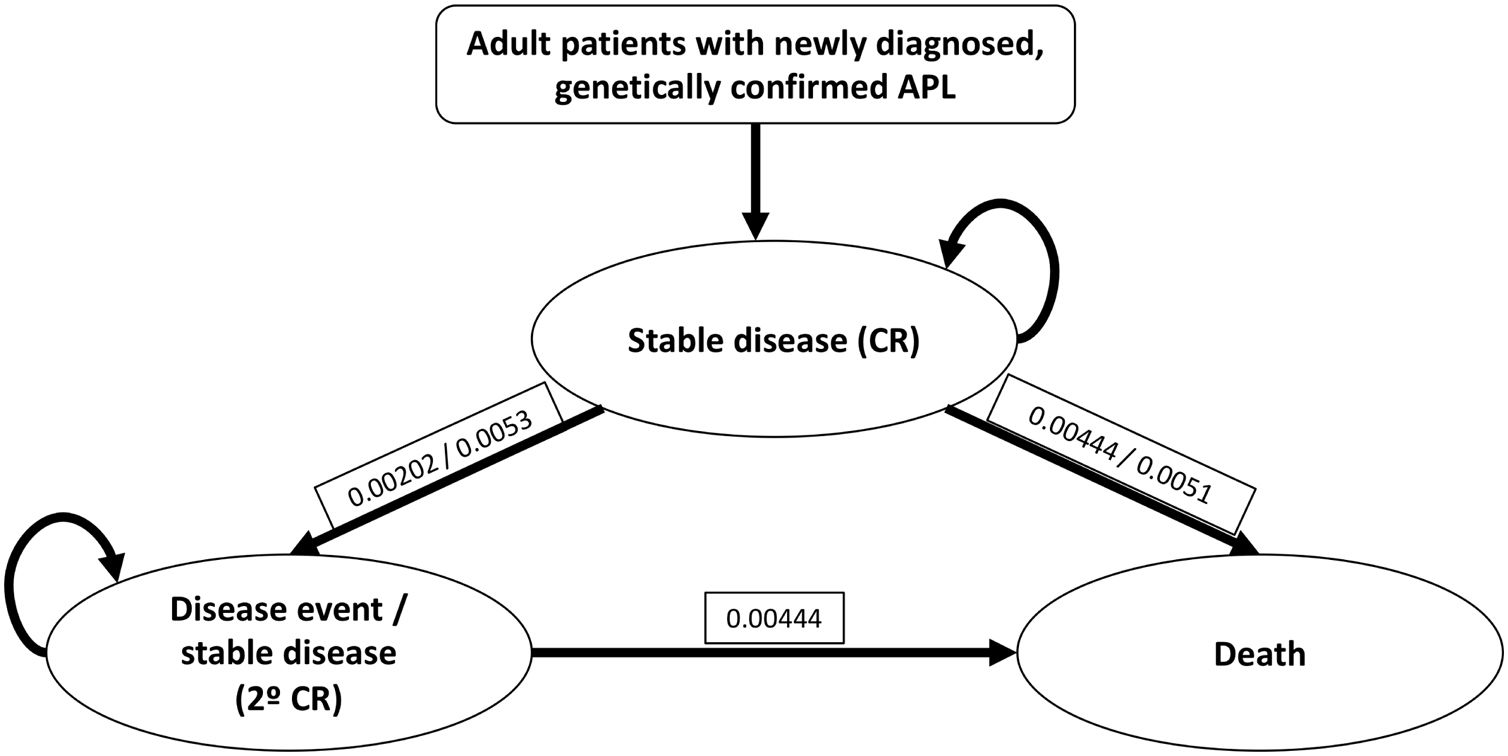
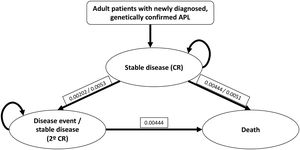
Patients and methodsModel structureWe performed a three-state Markov analysis: stable disease, disease event and death. Stable disease state comprised of first-line response and CR, the disease event state included relapse, second-line response and second CR. We used the available data from Tallman et al., with an error correction factor of 0.78 per year for stable disease, and of 0.65 per year for the disease event state.14 Each patient entered the model during the stable disease event, with transitions occurring each month to prove the probability of moving from stable disease to disease event or death. After a disease event, initial treatment was discontinued and second-line treatment started (Figure 1).
Three-state Markov model for APL treatment with the modified IC-APL protocol.4,14
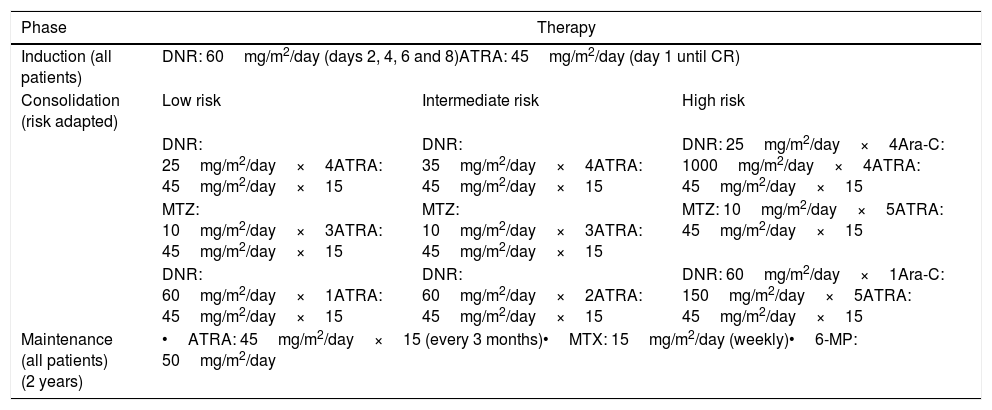
The modified IC-APL protocol is specified in the Crespo-Solis study and is composed of three phases: (1) an induction phase (ATRA plus DNR); (2) a consolidation phase (ATRA plus DNR, Ara-C and MTZ), and (3) a maintenance phase (ATRA plus MTX and 6-MP) (Table 1), diverting from the IC-APL in the administration of prophylactic intrathecal chemotherapy to patients considered to be at high risk.15,16
Modified IC-APL 2006 protocol regimen.15
| Phase | Therapy | ||
|---|---|---|---|
| Induction (all patients) | DNR: 60mg/m2/day (days 2, 4, 6 and 8)ATRA: 45mg/m2/day (day 1 until CR) | ||
| Consolidation (risk adapted) | Low risk | Intermediate risk | High risk |
| DNR: 25mg/m2/day×4ATRA: 45mg/m2/day×15 | DNR: 35mg/m2/day×4ATRA: 45mg/m2/day×15 | DNR: 25mg/m2/day×4Ara-C: 1000mg/m2/day×4ATRA: 45mg/m2/day×15 | |
| MTZ: 10mg/m2/day×3ATRA: 45mg/m2/day×15 | MTZ: 10mg/m2/day×3ATRA: 45mg/m2/day×15 | MTZ: 10mg/m2/day×5ATRA: 45mg/m2/day×15 | |
| DNR: 60mg/m2/day×1ATRA: 45mg/m2/day×15 | DNR: 60mg/m2/day×2ATRA: 45mg/m2/day×15 | DNR: 60mg/m2/day×1Ara-C: 150mg/m2/day×5ATRA: 45mg/m2/day×15 | |
| Maintenance (all patients) (2 years) | •ATRA: 45mg/m2/day×15 (every 3 months)•MTX: 15mg/m2/day (weekly)•6-MP: 50mg/m2/day | ||
DNR: daunorubicin; ATRA: all-trans retinoic acid; CR: complete remission; DEX: dexamethasone; BID: twice a day; WBC: white blood cell count; PLTs: platelets; Ara-C: cytarabine; MTZ: mitoxantrone; MTX: methotrexate; 6-MP: 6-mercaptopurine.
We included all of the newly diagnosed patients with APL, either by cytogenetic or molecular studies from peripheral blood smear or bone marrow sample, treated with the modified IC-APL protocol as a first-line option, in the period spanning 2007–2013 at the National Institute of Health Sciences and Nutrition Salvador Zubirán in Mexico City.
Clinical characteristics and survival parametersRegarding clinical characteristics, we considered gender, age and risk at diagnosis, based on international criteria.17 Risk stratification followed the recommendations of Sanz et al., considering patients at low risk those with a white blood cell (WBC) count <10×109/L and a platelet count >40×109/L, at intermediate risk those with a WBC<10×109/L and a platelet count <40×109/L and at high risk those with a WBC≥10×109/L.16,18 Concerning survival parameters, definition of CR rates, disease-free survival (DFS) and OS were considered the same as those in Crespo-Solís et al.15 For the ATRA plus ATO and ATRA plus idarubicin (IDA) protocols, we considered the data available from Lo-Coco et al.7 and for the ATRA plus Ara-C or DNR protocol, we considered the data from Ades et al. and Powell et al.19,20
Cost-effectiveness and cost-utility analysesChemotherapy costs were obtained from the Hematology & Oncology Department, with the aid of the Social Service Department. Medical costs from our Institute were obtained from the internal cost table, considering the institutional perspective cost (without a subsidy). The average number of years lived estimate and the quality-adjusted life-years (QALY) were obtained according to the recommendations made by Shen et al., using the area under the Kaplan-Meier curve.21
For the CEA, the CR was compared with the cost for each protocol and then compared by using the incremental cost-effectiveness ratios (ICERs) and for the CUA, the QALYs were compared with the cost for each protocol and then compared by using the incremental cost-utility ratios (ICURs).22 The formulas used for the ICER and ICUR are presented as follows:
where A corresponds to the comparator study (ATRA plus ATO, ATRA plus IDR or ATRA plus Ara-C and DNR protocol) and B corresponds to the compared study (IC-APL).ResultsClinical characteristics and survival parametersOf the 20 patients included in our study, 14 (70%) were women who had a median age of 38 years (range 17–79 years) at the time of diagnosis. Risk-stratification was as follows: 5 patients (25%) were classified as low-risk, 12 (60%), as intermediate-risk and 3 (15%), as high-risk.
CR was achieved in 18 patients (90%) treated with the modified IC-APL protocol as a first-line option and the remaining two patients (10%) had different outcomes: one died in induction, while the other one never achieved a response. Of the patients that achieved CR, 1 relapsed (5.5%) and later died after the second relapse. The mean estimated OS was 89 months (CI 95%, 86.61–101.51), with 89% of the patients alive at 39 months and the mean estimated disease-free survival (DFS) was 91 months (CI 95%, 83.71–99.81), with 94% of patients free of disease at 24 months.
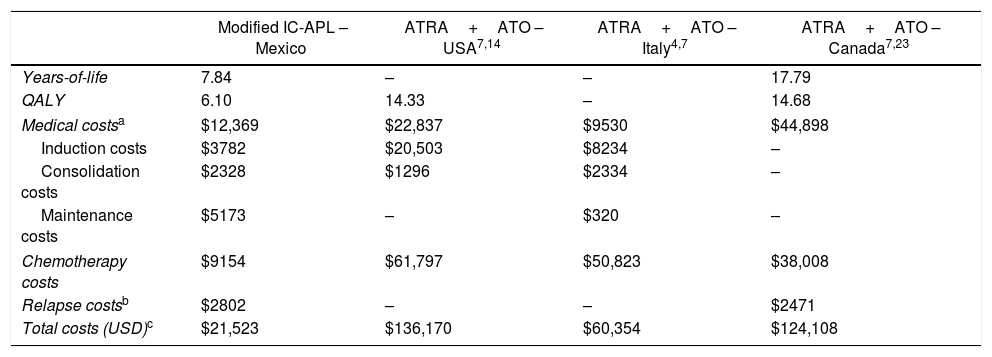
Cost-effectiveness and cost-utility analysesThe median treatment cost of the IC-APL protocol was $21,523 USD. The median length of treatment was 29 months (range, 27–39 months), with a median of life without treatment of 41 months (0–68 months). The average number of years-of-life in our study was 7.84 years, with the average QALY of 6.1 The total cost of ATRA+ATO in Italy was $60,354 USD, in Canada, $124,108 USD (QALY of 14.68) and in the USA, $136,170 USD (QALY of 14.33) (Table 2).
Comparison of costs in the treatment of APL.
| Modified IC-APL – Mexico | ATRA+ATO – USA7,14 | ATRA+ATO – Italy4,7 | ATRA+ATO – Canada7,23 | |
|---|---|---|---|---|
| Years-of-life | 7.84 | – | – | 17.79 |
| QALY | 6.10 | 14.33 | – | 14.68 |
| Medical costsa | $12,369 | $22,837 | $9530 | $44,898 |
| Induction costs | $3782 | $20,503 | $8234 | – |
| Consolidation costs | $2328 | $1296 | $2334 | – |
| Maintenance costs | $5173 | – | $320 | – |
| Chemotherapy costs | $9154 | $61,797 | $50,823 | $38,008 |
| Relapse costsb | $2802 | – | – | $2471 |
| Total costs (USD)c | $21,523 | $136,170 | $60,354 | $124,108 |
IC-APL: International Consortium on Acute Promyelocytic Leukemia; ATRA+ATO: all-trans retinoic acid+arsenic trioxide; QALY: quality-adjusted life-years; USD: US dollars.
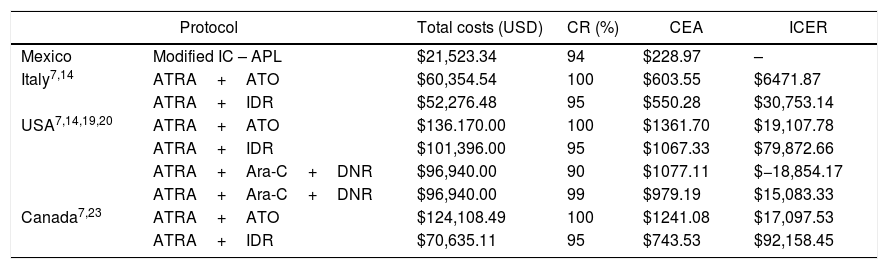
The CEA yielded $227, $603, $1241 and $1361 USD per percentage of CR in Mexico (IC-APL), Italy (ATRA plus ATO), Canada (ATRA plus ATO) and the USA (ATRA plus ATO), respectively. When comparing the ICER between the IC-APL and the ATRA plus ATO protocols, we found a difference of $6497, $19,133 and $17,123 USD in Italy, the USA and Canada, respectively (Table 3).
Cost-effectiveness analysis in the treatment of APL.
| Protocol | Total costs (USD) | CR (%) | CEA | ICER | |
|---|---|---|---|---|---|
| Mexico | Modified IC – APL | $21,523.34 | 94 | $228.97 | – |
| Italy7,14 | ATRA+ATO | $60,354.54 | 100 | $603.55 | $6471.87 |
| ATRA+IDR | $52,276.48 | 95 | $550.28 | $30,753.14 | |
| USA7,14,19,20 | ATRA+ATO | $136.170.00 | 100 | $1361.70 | $19,107.78 |
| ATRA+IDR | $101,396.00 | 95 | $1067.33 | $79,872.66 | |
| ATRA+Ara-C+DNR | $96,940.00 | 90 | $1077.11 | $−18,854.17 | |
| ATRA+Ara-C+DNR | $96,940.00 | 99 | $979.19 | $15,083.33 | |
| Canada7,23 | ATRA+ATO | $124,108.49 | 100 | $1241.08 | $17,097.53 |
| ATRA+IDR | $70,635.11 | 95 | $743.53 | $92,158.45 | |
USD: US dollars; CR: complete remission; CEA: cost-effectiveness analysis; ICERs: incremental cost-effectiveness ratios; IC-APL: International Consortium on Acute Promyelocytic Leukemia; ATRA+ATO: all-trans retinoic acid+arsenic trioxide; ATRA+IDR: all-trans retinoic acid+idarubicin; ATRA+Ara-C+DNR: all-trans retinoic acid+cytarabine+daunorubicin.
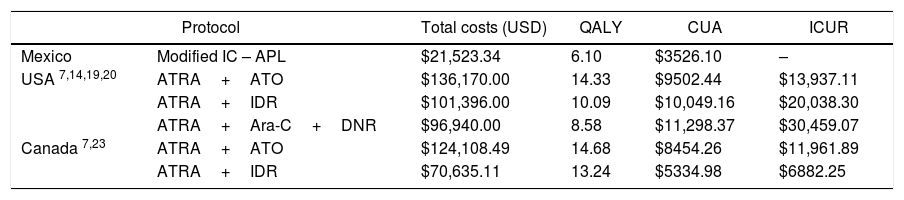
In relation to the CUA, each QALY gained in Mexico costs $3500 USD, in Canada, $8, 454 USD and in the USA, $9502 USD. When comparing the ICUR between the IC-APL and the ATRA plus ATO protocols, we found a difference in cost of $13,955 and of $11,979 USD in the USA and Canada, respectively (Table 4).
Cost-utility analysis in the treatment of APL.
| Protocol | Total costs (USD) | QALY | CUA | ICUR | |
|---|---|---|---|---|---|
| Mexico | Modified IC – APL | $21,523.34 | 6.10 | $3526.10 | – |
| USA 7,14,19,20 | ATRA+ATO | $136,170.00 | 14.33 | $9502.44 | $13,937.11 |
| ATRA+IDR | $101,396.00 | 10.09 | $10,049.16 | $20,038.30 | |
| ATRA+Ara-C+DNR | $96,940.00 | 8.58 | $11,298.37 | $30,459.07 | |
| Canada 7,23 | ATRA+ATO | $124,108.49 | 14.68 | $8454.26 | $11,961.89 |
| ATRA+IDR | $70,635.11 | 13.24 | $5334.98 | $6882.25 | |
USD: US dollars; QALY: quality-adjusted life-years; CUA: cost-utility analysis; ICURs: incremental cost-utility ratios; IC-APL: International Consortium on Acute Promyelocytic Leukemia; ATRA+ATO: all-trans retinoic acid+arsenic trioxide; ATRA+IDR: all-trans retinoic acid+idarubicin; ATRA+Ara-C+DNR: all-trans retinoic acid+cytarabine+daunorubicin.
In our study conducted at a tertiary referral center in Mexico City, we found that the total median cost of the APL treatment, using the modified IC-APL protocol, was $21,523 USD, with a 94% CR and a CEA of $227 USD (per each percentage of CR). Medical costs in our study represented most of the total median cost of treatment (57%). The ICER and ICUR were lower compared to Italy, the USA and Canada for the former, and the USA and Canada for the latter, since the Italian report did not include their QALY.
According to this study, although ATRA plus ATO regimen patients have fewer clinical adverse events and shorter duration of treatments, compared to regimens with ATRA plus chemotherapy, which lead to lower medical costs, the medical costs of the modified IC-APL protocol were higher than Italy's, but lower than USA's and Canada's ($12,369 USD, $9530 USD, $22,837 USD and $44,898 USD, respectively).4,14,23 Additionally, hospitalization during induction with the modified IC-APL protocol is longer, when compared with to ATRA plus ATO, leading to higher medical costs in Mexico, compared to the reported costs in Italy.
Chemotherapy costs of the modified IC-APL protocol in Mexico represented 42% of the total median costs and were found to be lower, compared to those reported in the USA, Italy, and Canada (85.2, 82 and 75.9%, respectively).4,14,23 Access to the drugs in the modified IC-APL protocol is easy, contrary to ATO, whose estimated cost ($391 USD for each 10-mg vial) renders it more difficult to attain.23 In addition, the current recommendations include the use of ATRA plus ATO only for low-to-intermediate-risk patients, as comparative economic studies from Canada and Italy did not include high-risk patients.4,14,23 Even considering that 15% of our patients were high-risk patients and received Ara-C during consolidation, chemotherapy costs were found to be lower, compared to these countries. Regarding the incremental cost-effectiveness ratios (ICERs), in order to achieve the same CR rates of the ATRA plus ATO protocols, an increase of $6497 to $19,133 USD would be required.4,14,23 Concerning the comparison of the ICER to the ATRA plus chemotherapy protocols, an even higher cost is represented, since the CR rates are similar.7,19,20
In spite of our results reporting lower QALY, when compared to those of the USA (6.1 years vs 14.33 and 8.58 years in the ATRA plus ATO and ATRA plus chemotherapy protocols, respectively) and of Canada (14.58 years in the ATRA plus ATO protocol), the cost-utility index of the modified IC-APL protocol is the lowest ($3500 USD pear QALY). These results indicate that for each QALY, an estimated cost of $3500, $9502 and $8454 USD is required in Mexico, the USA and Canada, respectively. According to the incremental cost-utility ratios (ICURs), in order to obtain the same QALY of the ATRA plus ATO protocols, an increase of $11,979 to $13,955 USD would be required.14,23
This study has several limitations. First, all medical visits (hematologist or other specialists) are not routinely charged for at our institution, consequently contributing to lower medical costs during patient hospitalizations. Second, the years of analysis among studies used for comparison were different and could probably have derived a cost difference due to the inflation rate. Third, patients included in this analysis were evaluated and treated from 2007 to 2013, when the exchange rate of pesos to dollars ranged from $9.1 to $15.23, but we used a more representative exchange rate of 20.76 (from November 12, 2016) to present updated costs. However, to overcome these limitations, this model was based on data taken directly from medical records for each individual, which renders highly accurate medical and chemotherapy costs.
Estimating the financial impact of including all drugs of the modified IC-APL treatment regimen on the drugs supplied by the national public health system is critical to understand the net benefit to clinical outcomes and QALYs. Further research is needed to establish the cost-effectiveness at other referral centers in our country to standardize treatment and analyze the benefit of the IC-APL in other hospital settings. Considering the similar response rates among treatment protocols, the lower cost and the easy access to the modified IC-APL regime in settings, such as Mexico, this regimen is a cost-effective and cost-useful protocol that should be considered for all patients recently diagnosed with APL.
Meeting of ethical standardsThis study was approved by our local Ethics Committee, as was the original study by Crespo-Solís et al., which complied with the principles established in the Declaration of Helsinki for research involving human subjects.
Conflicts of interestThe authors declare no conflicts of interest.