The COVID-19 pandemic has pushed the world towards social, economic, and medical challenges. Scientific research in medicine is the only means to overcome novel and complex diseases like COVID-19. To sum up the therapeutic wild-goose chase, many available antivirals and repurposed drugs have failed to show successful clinical evidence in patient recovery, several vaccine candidates are still waiting in the trial pipelines and a few have become available to the common public for administration in record time.
However, with upcoming evidence of coronavirus mutations, available vaccines may thrive on the spirit of doubt about efficacy and effectiveness towards these new strains of Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV2). In all these collective uncertainties, plasma therapy has shown a ray of hope for critically ill patients.
To date, with very few published case studies of convalescent plasma in COVID-19, there are two school of thought process in the scientific community regarding plasma therapy efficiency and this leads to confusion due to the lack of optimal randomized and controlled studies.
Without undertaking any robust scientific studies, evidence or caution, accepting any therapy unanimously may cause more harm than good, but with a clearer understanding of SARS-CoV2 immunopathology and drug response, plasma therapy might be the silver lining against COVID-19 for the global community.
Globally, as January 4, 2021, there have been 83,910,386 confirmed COVID-19 cases, including 1839,660 deaths, as per World Health Organization (WHO) reports.1 Although the race to find any substantial COVID-19 treatment and effective vaccine has gained a necessary acceleration, with a steep increase in the infection rate worldwide, the majority of the countries are currently on the doorstep of a second wave of COVID-19. Even after one year from the novel coronavirus (SARS-CoV2) detection in China,2 therapeutic strategies for novel coronavirus disease 2019 (COVID-19) with antivirals and repurposed drugs are facing critical challenges for approval due to the lack of clinical evidence or inadequate successful in vivo patient reports. Meanwhile, the sprint to produce safe and effective vaccines has gained tremendous momentum with a few candidates already in the market,3 but their success is still uncertain . Recently, the US Food and Drug Administration (FDA) and American Association of Blood Banks have asked vaccine recipients to hold off plasma donation, owing to antibody selectivity towards specific regions of SARS-CoV2.4 This further raises questions on vaccine efficiency in the case of strain mutations of COVID-19-causing coronavirus.5,6
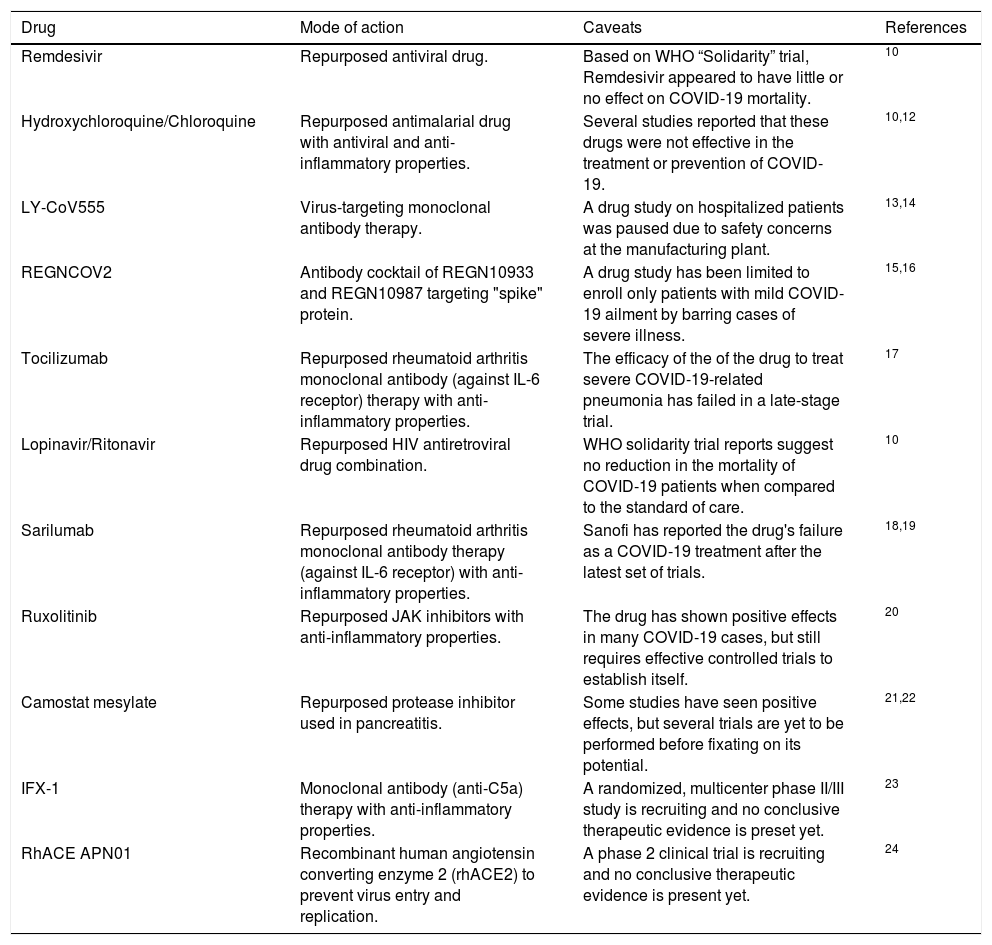
Since COVID-19 therapeutic research began, with available knowledge of the last two decades against two different strains of beta-coronaviruses (βCoVs): Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV),7,8 researchers rushed to repurpose existing drugs in an attempt to alter the course of severe COVID-19, but the results were far less than clinically significant. A list of drugs9 used against COVID-19 for therapeutic purpose have been reported in Table 1. The Solidarity Trial evaluation report published by the WHO on October 15, 2020, reveals interim results of experimental antiviral Remdesivir, the malaria medication Hydroxychloroquine, a combination of HIV drugs Lopinavir/Ritonavir and an immune system messenger Interferon treatments had little or no effect on overall mortality, initiation of ventilation and duration of hospital stay in hospitalized patients, as these drugs showed health risks and failed to reduce COVID-19 mortality in patient cohorts.10 Amidst the wait for effective treatment, passive immunization strategy of convalescent plasma therapy emerged as a hopeful tactic for the prevention and management of COVID-19, but some convalescent plasma studies, such as the multi-centric study funded by the Indian Council of Medical Research (ICMR),11 have found no significant clinical improvement or reduction in mortality in severe COVID-19 cases. This adds to more confusion about the use of plasma therapy in COVID-19. The present review aims to provide an overview of the recent plasma therapy research in the COVID-19 domain, while evaluating critical limitations to enhance clinical efficacy.
COVID-19 drugs used for therapeutic purpose.
| Drug | Mode of action | Caveats | References |
|---|---|---|---|
| Remdesivir | Repurposed antiviral drug. | Based on WHO “Solidarity” trial, Remdesivir appeared to have little or no effect on COVID-19 mortality. | 10 |
| Hydroxychloroquine/Chloroquine | Repurposed antimalarial drug with antiviral and anti-inflammatory properties. | Several studies reported that these drugs were not effective in the treatment or prevention of COVID-19. | 10,12 |
| LY-CoV555 | Virus-targeting monoclonal antibody therapy. | A drug study on hospitalized patients was paused due to safety concerns at the manufacturing plant. | 13,14 |
| REGNCOV2 | Antibody cocktail of REGN10933 and REGN10987 targeting "spike" protein. | A drug study has been limited to enroll only patients with mild COVID-19 ailment by barring cases of severe illness. | 15,16 |
| Tocilizumab | Repurposed rheumatoid arthritis monoclonal antibody (against IL-6 receptor) therapy with anti-inflammatory properties. | The efficacy of the of the drug to treat severe COVID-19-related pneumonia has failed in a late-stage trial. | 17 |
| Lopinavir/Ritonavir | Repurposed HIV antiretroviral drug combination. | WHO solidarity trial reports suggest no reduction in the mortality of COVID-19 patients when compared to the standard of care. | 10 |
| Sarilumab | Repurposed rheumatoid arthritis monoclonal antibody therapy (against IL-6 receptor) with anti-inflammatory properties. | Sanofi has reported the drug's failure as a COVID-19 treatment after the latest set of trials. | 18,19 |
| Ruxolitinib | Repurposed JAK inhibitors with anti-inflammatory properties. | The drug has shown positive effects in many COVID-19 cases, but still requires effective controlled trials to establish itself. | 20 |
| Camostat mesylate | Repurposed protease inhibitor used in pancreatitis. | Some studies have seen positive effects, but several trials are yet to be performed before fixating on its potential. | 21,22 |
| IFX-1 | Monoclonal antibody (anti-C5a) therapy with anti-inflammatory properties. | A randomized, multicenter phase II/III study is recruiting and no conclusive therapeutic evidence is preset yet. | 23 |
| RhACE APN01 | Recombinant human angiotensin converting enzyme 2 (rhACE2) to prevent virus entry and replication. | A phase 2 clinical trial is recruiting and no conclusive therapeutic evidence is present yet. | 24 |
Convalescent plasma therapy can be traced back to the 20th century, when the plasma of animals, stimulated with infectious agents, were used for immediate short-term antibody-based immunization for the prevention and treatment of diseases.25 Kitaso and Von Behring26 achieved a significant milestone in the 1890s for treating diphtheria using blood serum from immunized animal donors, which were later replaced by plasma from recovered donors with specific antibody-based humoral immunity of human origin. Although antibiotics have superseded the requirement for plasma therapy in the case of bacterial infections,27 it is considered as an important strategy against viral pathogens when prophylaxis or treatments are not available. Besides diphtheria, plasma therapy has also been used in the prophylaxis and treatment of several bacterial diseases, such as pertussis and scarlet fever.28 For the first time, during the Spanish flu pandemic of 1918, plasma therapy was reported to be majorly effective in the case of viral diseases on a mass scale29,30 and soon, extensive research on convalescent plasma therapy indicated significant efficacy in the case of chickenpox, measles, cytomegalovirus infection, H1N1 and H5N1 flu, parovirus B19 infection, Ebola, SARS and MERS.31–37
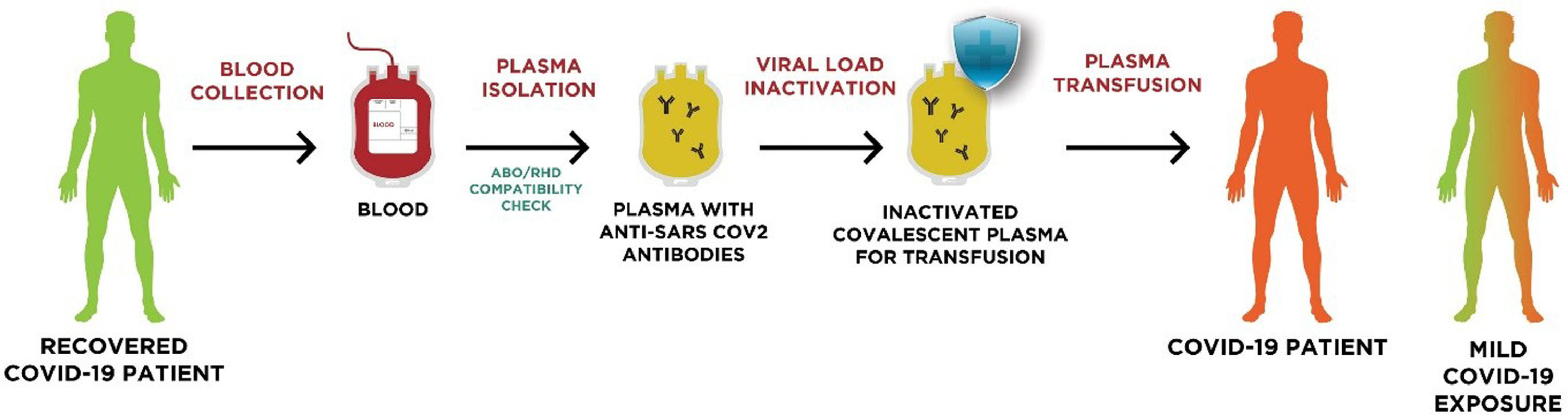
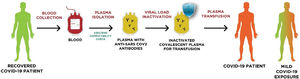
In convalescent plasma therapy, blood is isolated from a recovered patient who has developed humoral immunity against a particular disease-causing pathogen (e.g., SARS-CoV-2, in the case of COVID-19) and plasma containing specific neutralizing antibodies38 is obtained by apheresis for transfusion in diseased patients after matching the ABO compatibility (Figure 1). Besides neutralizing antibodies against the specific pathogen, the plasma obtained by apheresis also contains anti-inflammatory cytokines, clotting factors and other natural antibodies to support the immunomodulatory benefits of convalescent plasma therapy.39
Plasma Therapy Procedure for COVID-19 Treatment: The blood of a recovered COVID-19 patient is collected and convalescent plasma is recovered by apheresis. The colleted plasma undergoes an ABO/RHD compatibility check and a viral load inactivation before being processed for transfusion into a COVID-19 patient with severe/mild disease conditions. Adapted from Casadevall et al.40. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As the current pandemic scenario strictly deals with coronavirus, it is particularly eminent to discuss the therapeutic evidence of convalescent plasma therapy in the context of coronavirus-caused SARS and MERS diseases (Table 2).
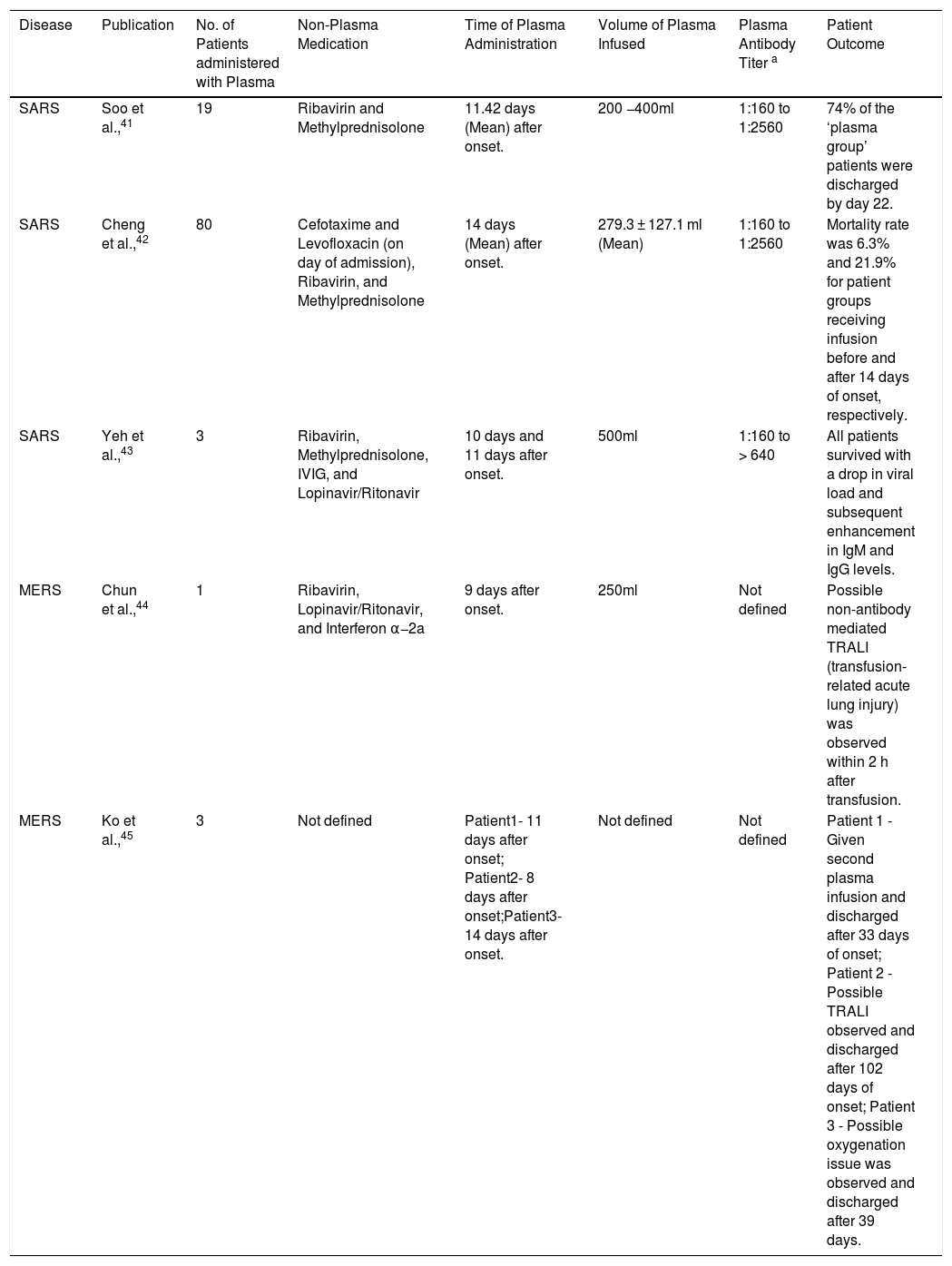
Plasma therapy reports of SARS and MERS.
| Disease | Publication | No. of Patients administered with Plasma | Non-Plasma Medication | Time of Plasma Administration | Volume of Plasma Infused | Plasma Antibody Titer a | Patient Outcome |
|---|---|---|---|---|---|---|---|
| SARS | Soo et al.,41 | 19 | Ribavirin and Methylprednisolone | 11.42 days (Mean) after onset. | 200 −400ml | 1:160 to 1:2560 | 74% of the ‘plasma group’ patients were discharged by day 22. |
| SARS | Cheng et al.,42 | 80 | Cefotaxime and Levofloxacin (on day of admission), Ribavirin, and Methylprednisolone | 14 days (Mean) after onset. | 279.3 ± 127.1 ml (Mean) | 1:160 to 1:2560 | Mortality rate was 6.3% and 21.9% for patient groups receiving infusion before and after 14 days of onset, respectively. |
| SARS | Yeh et al.,43 | 3 | Ribavirin, Methylprednisolone, IVIG, and Lopinavir/Ritonavir | 10 days and 11 days after onset. | 500ml | 1:160 to > 640 | All patients survived with a drop in viral load and subsequent enhancement in IgM and IgG levels. |
| MERS | Chun et al.,44 | 1 | Ribavirin, Lopinavir/Ritonavir, and Interferon α−2a | 9 days after onset. | 250ml | Not defined | Possible non-antibody mediated TRALI (transfusion-related acute lung injury) was observed within 2 h after transfusion. |
| MERS | Ko et al.,45 | 3 | Not defined | Patient1- 11 days after onset; Patient2- 8 days after onset;Patient3- 14 days after onset. | Not defined | Not defined | Patient 1 - Given second plasma infusion and discharged after 33 days of onset; Patient 2 - Possible TRALI observed and discharged after 102 days of onset; Patient 3 - Possible oxygenation issue was observed and discharged after 39 days. |
In the case of COVID-19, plasma therapy can act as an anti-viral, as well as an anti-inflammatory, agent against SARS-CoV2 infection, which may adversely affect pulmonary capacity and cause acute respiratory distress syndrome-like symptoms due to cytokine storms in severe stages.46,47
In a study at Prince of Wales Hospital in Hong Kong,42 80 SARS-CoV-infected patients were administered with convalescent plasma when their condition was not improving, even after receiving their methylprednisolone dose. The patient outcomes showed positive improvement in the health conditions, but it was observed that patients who received plasma therapy within 14 days from the infection showed better outcomes, compared to the patients who received plasma after 14 days. In the same hospital, another study41 was conducted to compare the effects of plasma therapy against methylprednisolone. Out of 40 SARS patients undergoing ribavirin and methylprednisolone treatment, 19 were administered with convalescent plasma, whereas 21 were given an additional dose of methylprednisolone. The group of patients who were treated with plasma therapy showed better recovery and reduced mortality. In a different study in Taiwan,43 3 critical SARS patients were administered with convalescent plasma and showed stark improvements within 24 h of the administration. Although the reported cases emphasized the success of plasma therapy in the SARS-CoV infection, the major limitation was the randomization and sample size issues. The inadequate, yet significant, success reports of convalescent plasma therapy further prompted researchers to use it as a therapeutic tool against the MERS-CoV infection. In a 2016 report,44 a MERS-CoV infected patient showed viral load reduction upon treatment with convalescent plasma therapy after administration of ribavirin and lopinavir/ritonavir with interferon α−2a dosage. In another small study,45 3 MERS patients were administered with convalescent plasma of different titers to determine the earliest seroconversion. Although all the patients recovered and were discharged from the hospital, the study reported that a higher neutralizing antibody titer (≥ 1:80) might be responsible for early seroconversion, thereby having a major impact on the immunity.
Several observational studies in COVID-19 patients using plasma therapy have shown positive results with some incidence of adverse effects (Table 3). The first press-published report of treating COVID-19 patients with convalescent plasma therapy came out in China, where 245 patients were treated with specific neutralizing antibodies and the therapy was assured to be safe and effective.48 Following this, a pilot study from China reported significant viral load reduction and improvements in the clinical symptoms for 10 severe COVID-19 patients, who were infused with convalescent plasma as an addition to antivirals and supportive care.49 In another small study, 5 COVID-19 patients on mechanical ventilation were infused with a neutralizing plasma titer > 1:1000 and showed significant reduction in fever, viral load and ARDS (acute respiratory distress syndrome).50 Another patient study in China51 also portrayed similar benefits of convalescent plasma therapy and hailed it as a potential solution to the pandemic crisis. Amidst these single-arm studies, one particular study by Wu et al.,52 reported that anti-SARS-CoV2 spike-binding antibodies in patients were detected from days 10 to15 after the disease onset and hinted at the need to explore the interplay between viral load and immune response. The study also reported that elderly and middle-aged COVID-19 patients had significantly higher anti-SARS-CoV2 antibody titers than young patients. In another study, Zhang et al. reported plasma therapy benefits in COVID-19 patients with a history of severe underlying health conditions, such as hypertension, renal failure, ARDS and chronic obstructive pulmonary disease (COPD). They further suggested that patients who survive severe illness might have higher anti-SARS-CoV2 antibody titers with longer persistence, compared to patients with non-severe disease.53 Along the same lines, a study by Zhao et al., concluded that a higher antibody titer in patients is independently associated with the severity of the disease and worse clinical stages.54
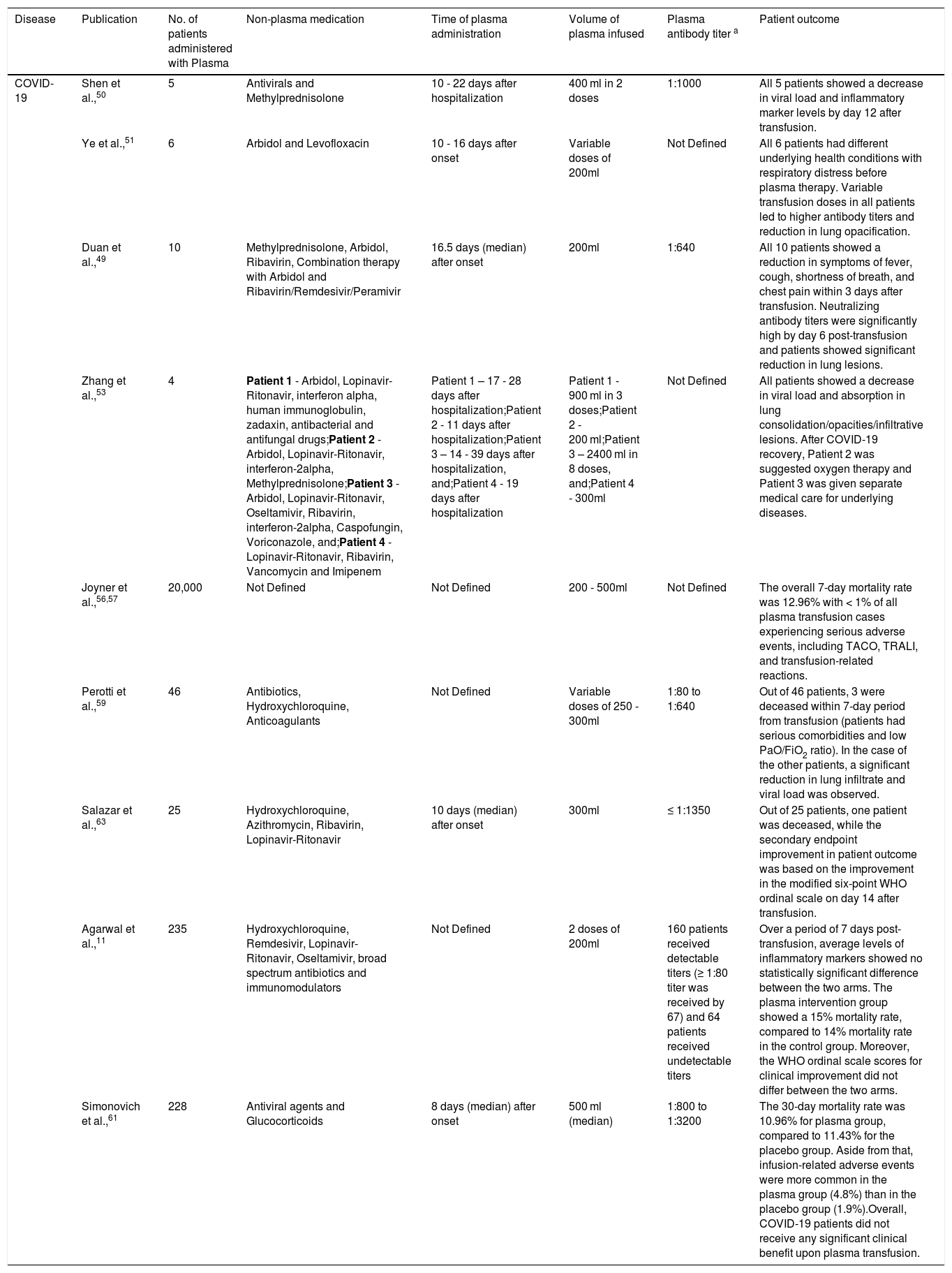
Plasma therapy reports on COVID-19.
| Disease | Publication | No. of patients administered with Plasma | Non-plasma medication | Time of plasma administration | Volume of plasma infused | Plasma antibody titer a | Patient outcome |
|---|---|---|---|---|---|---|---|
| COVID-19 | Shen et al.,50 | 5 | Antivirals and Methylprednisolone | 10 - 22 days after hospitalization | 400 ml in 2 doses | 1:1000 | All 5 patients showed a decrease in viral load and inflammatory marker levels by day 12 after transfusion. |
| Ye et al.,51 | 6 | Arbidol and Levofloxacin | 10 - 16 days after onset | Variable doses of 200ml | Not Defined | All 6 patients had different underlying health conditions with respiratory distress before plasma therapy. Variable transfusion doses in all patients led to higher antibody titers and reduction in lung opacification. | |
| Duan et al.,49 | 10 | Methylprednisolone, Arbidol, Ribavirin, Combination therapy with Arbidol and Ribavirin/Remdesivir/Peramivir | 16.5 days (median) after onset | 200ml | 1:640 | All 10 patients showed a reduction in symptoms of fever, cough, shortness of breath, and chest pain within 3 days after transfusion. Neutralizing antibody titers were significantly high by day 6 post-transfusion and patients showed significant reduction in lung lesions. | |
| Zhang et al.,53 | 4 | Patient 1 - Arbidol, Lopinavir-Ritonavir, interferon alpha, human immunoglobulin, zadaxin, antibacterial and antifungal drugs;Patient 2 - Arbidol, Lopinavir-Ritonavir, interferon-2alpha, Methylprednisolone;Patient 3 - Arbidol, Lopinavir-Ritonavir, Oseltamivir, Ribavirin, interferon-2alpha, Caspofungin, Voriconazole, and;Patient 4 - Lopinavir-Ritonavir, Ribavirin, Vancomycin and Imipenem | Patient 1 – 17 - 28 days after hospitalization;Patient 2 - 11 days after hospitalization;Patient 3 – 14 - 39 days after hospitalization, and;Patient 4 - 19 days after hospitalization | Patient 1 - 900 ml in 3 doses;Patient 2 - 200 ml;Patient 3 – 2400 ml in 8 doses, and;Patient 4 - 300ml | Not Defined | All patients showed a decrease in viral load and absorption in lung consolidation/opacities/infiltrative lesions. After COVID-19 recovery, Patient 2 was suggested oxygen therapy and Patient 3 was given separate medical care for underlying diseases. | |
| Joyner et al.,56,57 | 20,000 | Not Defined | Not Defined | 200 - 500ml | Not Defined | The overall 7-day mortality rate was 12.96% with < 1% of all plasma transfusion cases experiencing serious adverse events, including TACO, TRALI, and transfusion-related reactions. | |
| Perotti et al.,59 | 46 | Antibiotics, Hydroxychloroquine, Anticoagulants | Not Defined | Variable doses of 250 - 300ml | 1:80 to 1:640 | Out of 46 patients, 3 were deceased within 7-day period from transfusion (patients had serious comorbidities and low PaO/FiO2 ratio). In the case of the other patients, a significant reduction in lung infiltrate and viral load was observed. | |
| Salazar et al.,63 | 25 | Hydroxychloroquine, Azithromycin, Ribavirin, Lopinavir-Ritonavir | 10 days (median) after onset | 300ml | ≤ 1:1350 | Out of 25 patients, one patient was deceased, while the secondary endpoint improvement in patient outcome was based on the improvement in the modified six-point WHO ordinal scale on day 14 after transfusion. | |
| Agarwal et al.,11 | 235 | Hydroxychloroquine, Remdesivir, Lopinavir-Ritonavir, Oseltamivir, broad spectrum antibiotics and immunomodulators | Not Defined | 2 doses of 200ml | 160 patients received detectable titers (≥ 1:80 titer was received by 67) and 64 patients received undetectable titers | Over a period of 7 days post-transfusion, average levels of inflammatory markers showed no statistically significant difference between the two arms. The plasma intervention group showed a 15% mortality rate, compared to 14% mortality rate in the control group. Moreover, the WHO ordinal scale scores for clinical improvement did not differ between the two arms. | |
| Simonovich et al.,61 | 228 | Antiviral agents and Glucocorticoids | 8 days (median) after onset | 500 ml (median) | 1:800 to 1:3200 | The 30-day mortality rate was 10.96% for plasma group, compared to 11.43% for the placebo group. Aside from that, infusion-related adverse events were more common in the plasma group (4.8%) than in the placebo group (1.9%).Overall, COVID-19 patients did not receive any significant clinical benefit upon plasma transfusion. |
Convalescent plasma therapy gained an FDA expanded access status for the treatment of COVID-19 in April.55 As a part of the expanded access program, Joyner et al.,56 suggested beneficial effects of plasma transfusion in COVID-19 treatment along with incidence of related TRALI and transfusion-associated circulatory overload (TACO) complications in some patients. Extending the same open-label trial, plasma transfusion was performed with 20,000 patients and regarded safe with a very low incidence of adverse effects.57 Going a step ahead of using convalescent plasma, Perotti et al. utilized hyperimmune plasma (a concentrate obtained from a pool of convalescent plasma donors)58 to treat a group of 46 patients and showed significant reduction in the viral load and ARDS conditions for 42 of them.59
In May 2020, the ICMR approved 38 centers for an open-label randomized parallel-arm Phase II plasma transfusion study (PLACID trial) in COVID-19 patients.60 The study with 464 adults failed to show any significant association of convalescent plasma transfusion to reduction in disease progression and mortality, thus setting a major question on the utility of plasma therapy in COVID-19 treatment. Another recent study by Simonovich et al. also concluded on similar terms, as no significant difference was observed in COVID-19 clinical outcomes between the plasma therapy group and placebo group.61 Currently, the largest study (PLATINA trial) to date is ongoing to analyze the effectiveness of plasma therapy in approximately 500 COVID-19 patients and, although the ICMR study results have constituted a setback to the use of plasma transfusion, positive results of this Maharashtra (India) government-approved study might be the last hope for establishing plasma therapy as a standard therapeutic intervention in COVID-19 cases.62
DiscussionAmidst the critical search for medications and vaccines against COVID-19, the age-old technique of passive immunization using convalescent plasma emerged as a savior for several patients infected with SARS-CoV2. Many observational single-arm studies worldwide showed significant improvements in the clinical outcomes and mortality of severely ill COVID-19 patients by reducing viral load and lung injury,49–51 but some patient cohort studies11,61 reported no significant effect of plasma transfusion in determining patient conditions. Furthermore, a meta-analysis study by Chan et al. in 2020 reported uncertainty in determining the role of plasma therapy in reducing COVID-19 mortality, but suggested that it may increase the improvement of clinical symptoms by up to 15 days.64 These patient reports using plasma therapy have their own specific caveats to amend, which might result in more efficient ways of studying plasma transfusion in COVID-19 cases.
Most of the studies to date showed benefits of plasma transfusion in COVID-19 patient outcomes, but, as discussed in the review, they had the limitation of not being randomized and controlled. This factor was taken into consideration in the recently published PLACID trial report.11 Although the PLACID trial questioned the effectiveness of plasma transfusion in COVID-19 patients, aside from the already mentioned limitations in the paper, it had a major drawback: 94% of the donors had mild disease, while two-thirds of them had a median titer value of 1:40. The US FDA recommends the use of donor convalescent plasma with the 1:160 neutralizing titer65 and studies have shown that early intervention with higher neutralizing antibody titers66,67 show better clinical outcomes in COVID-19 patients. Therefore, including these considerations in the PLACID trial might have shown significant results in patient outcomes. Moreover, the PLACID trial does not mention the use of hyperimmune plasma therapy,58 though the donor titers were considerably lower than the recommended levels. Utilizing the technology of hyperimmune plasma therapy in the PLACID trial also might have led to a positive perspective, as higher neutralizing antibody titers in transfused plasma would have shown better therapeutic efficiency.
The above-discussed drawbacks of non-standardized neutralizing antibody titers and plasma transfusion conditions are crucial concerns that impede the potential practice of plasma therapy against COVID-19. Aside from these limitations, our review also sheds light on three important points of concern in the case of utilizing plasma therapy for patients with severe COVID-19:
- 1.
In the case of severe COVID-19, an inflammatory cytokine storm has been observed in several studies,68 in addition to higher antibody titers.69 Reports have shown that Anti-SARS-CoV-IgG levels generally peak in the fourth week70 and this robust response not only contributes to the clearance of viral load, but also might lead to antibody dependent enhancement (ADE) of infection and immune-mediated tissue damage.54,69 Furthermore, a severe disease-mediated cytokine storm exhibits elevated pro-inflammatory cytokine levels which may lead to respiratory failure, tissue damage, and lymphopenia.71 Lymphopenia causes a low count of T cells in the body and this significant decrease further halts the immune action against the SARS-CoV2 viral antigen bound by antibodies. Previous studies have shown that the reduction of the inflammatory cytokine storm can oppose lymphopenia72 and therefore, resorting to immunomodulatory tactics before plasma transfusion in severe COVID-19 patients can enhance the efficiency of convalescent plasma therapy and reduce mortality. One of the frequently studied approaches is the dampening of inflammatory responses, specifically IL-6 (stable indicator for poor outcomes in ARDS and pneumonia),73 by using the monoclonal antibody (mAb) Tocilizumab.74 Other neutralizing mAbs against different potential inflammatory targets, such as IL-1β and TNF-α,75 or small molecule blockers of immunopathology-based downstream signaling might also be used for controlled immunosuppression. The second approach involves mesenchymal stem cells (MSCs) for their anti-inflammatory and anti-viral effects.76 Such immunomodulatory approaches will further reduce the risks of TRALI in COVID-19 patients undergoing plasma transfusion.77
- 2.
Although studies on COVID-19 have not yet shed significant light on antiviral drug resistance, there is a high risk of conventional antivirals being ineffective against SARS-CoV2, owing to previous records of antiviral resistance in single-stranded RNA viruses, such as hepatitis and influenza viruses.78 Mutations, or genetic changes, arise naturally in all viruses, including SARS-CoV-2, as they replicate and circulate in human populations. In the case of SARS-CoV-2, these mutations are accumulating at a rate of around one to two mutations per month globally.79 As a result of this, thousands of mutations might already have arisen in the SARS-CoV-2 genome since the virus emerged in 2019. Protein structure mutations have been observed to play a major role in drug resistance and, although studies on SARS-CoV2 drug resistance are yet to decipher specific resistance mechanisms, December 2020 witnessed a rapidly spreading mutated strain of Corona virus contributing to increased transmission.5,6 The new UK coronavirus variant VUI 202,012/01 and the South Africa coronavirus variant 501.V2, with genetic mutations in the “spike” protein and other alterations, could lead to loss of antibody affinity against SARS-CoV2. These new strains have already raised huge concerns on available vaccine efficiency and scientists are claiming that indiscriminate and random plasma transfusion might fuel more such mutations.80 These mutation concerns might also be due to the rampant use of antivirals without proper clinical data. Therefore, besides the immediate requirement for optimized controlled plasma therapy studies, research for detecting more drug targets should be heavily ongoing, besides opting for safer anti-viral options, such as mesenchymal stromal cells.81
- 3.
A critical point of concern in COVID-19 plasma therapy is based on the limitations of convalescent plasma collection procedure and processing. The safety and efficacy of convalescent plasma therapy for COVID-19 treatment is fairly dependent on technical, ethical and legal safeguards of transfusion (informed doctor-patient consent, compliance with regulatory requirements, effective pre-screening and pre-donation testing of candidates, etc.).82 To assure good quality of blood transfusion or plasma collection procedure, approval for blood banks and hospitals by regulatory authorities, mobilizing more technolegally-approved transfusion and apheresis machines, hiring trained medical professionals, etc., require mandatory attention, in addition to legal medical licensing.83 Aside from the procedure, the quality of the convalescent plasma is also a major criterion for ensuring plasma therapy efficiency. It is better to avoid whole blood transfusion, unless clinically indicated, as plasma collection by apheresis can optimize the generated plasma volume by reducing the loss of red blood cells. Moreover, viral inactivation and transfusion-transmitted infections testing (using nucleic acid testing (NAT) and enzyme-linked fluorescence assay (ELFA)) should be made compulsory in the donor screening process. These processing procedures significantly add to safe plasma collection, though donors can be excluded for unfavorable vein size or inadequate hemoglobin, even after passing the health screening for donation.84 Another crucial limiting factor in the collection and processing of plasma for COVID-19 treatment is the detection of neutralizing antibodies, as low neutralizing antibody titers may defeat the purpose of efficient plasma therapy even with optimum plasma volume and net donor antibody titers. Therefore, regulating and optimizing the quality of convalescent plasma collection and processing might accelerate plasma therapy towards being a more potential COVID-19 treatment.
As of now, with a vacuum of efficient anti-SARS-CoV2 medications, there is an alarming need for more randomized and controlled plasma therapy-based clinical studies on COVID-19 patient outcomes, with strong emphasis on the above-mentioned points of concern and drawbacks. While it is good to be hopeful, the question of convalescent plasma therapy being a silver lining in COVID-19 management is still a long way from being answered with a definite response, but with more research and better understanding of SARS-CoV2 immunopathology and drug response, plasma therapy might gain an unequivocal ‘thumbs-up’ from the global clinical community.
Conflict of interestThe authors declare no conflict of interest.