Paroxysmal nocturnal hemoglobinuria is a chronic, multi-systemic, progressive and life-threatening disease characterized by intravascular hemolysis, thrombotic events, serious infections and bone marrow failure. Paroxysmal nocturnal hemoglobinuria results from the expansion of a clone of hematopoietic cells that due to an inactivating mutation of the X-linked gene PIG-A are deficient in glycosylphosphatidylinositol-linked proteins. Early diagnosis, using flow cytometry performed on peripheral blood, the gold standard test to confirm the diagnosis of paroxysmal nocturnal hemoglobinuria, is essential for improved patient management and prognosis. The traditional therapy for paroxysmal nocturnal hemoglobinuria includes blood transfusion, anti-thrombosis prophylaxis or allogeneic bone marrow transplantation. The treatment that has recently become available is the complement blockade by the anti-C5 monoclonal antibody eculizumab. In this consensus, we are aiming to review the diagnosis and treatment of the paroxysmal nocturnal hemoglobinuria patients, as well as the early recognition of its systemic complications. These procedures express the opinions of experts and have been based on the best available evidence and international guidelines, with the purpose of increasing benefits and reducing harm to patients.
Paroxysmal nocturnal hemoglobinuria (PNH) is a chronic, multi-systemic, progressive and life-threatening disease characterized by intravascular hemolysis, thrombotic events, serious infections and bone marrow failure.1,2 Hemolysis in PNH is due to the action of the complement on abnormal red blood cells (RBCs). Compared with normal RBCs, PNH RBCs lyse more readily in the presence of the activated complement. Granulocytes and platelets are sensitive to the complement, as well. Two complement regulatory proteins, the CD55 (decay accelerating factor [DAF]) and CD59 (homologous restriction factor or membrane inhibitor of reactive lysis [MIRL]), were found to be missing from PNH blood cells, explaining the unusual sensitivity of RBCs to the hemolytic action of complement. The CD59, whose action is to inhibit the terminal complement cascade leading to the destruction of red blood cells may be more important.3–6 In the last decade, PNH has received novel and much more in-depth attention and a new kind of therapy has become available, the complement blockade by the anti-C5 monoclonal antibody eculizumab. In this consensus, we want to emphasize the diagnosis and treatment of the PNH patients, as well as the early recognition of its systemic complications.3–6
EpidemiologyPNH is an acquired clonal disorder of hematopoietic stem cells and its incidence is approximately 1–2 per million, with a prevalence ranging from 10 to 20 per million. The incidence rate in Great Britain/France is approximately 1.3 cases per million people7 and 0.13/100,000/year in Yorkshire (United Kingdom).8 PNH is chronic, progressive and life-threatening, diagnosed at any age (median age in the early to mid-thirties), affecting males and females equally and despite the best supportive care, it can have a significant impact on mortality, i.e., 5-year and 10-year mortality of 35% and approximately 50%, respectively1,7
PathophysiologyA multistep process seems necessary for PNH to develop. The acquired mutation in PNH occurs in the phosphatidylinositol glycan class A (PIGA) gene, which is responsible for the first step in the synthesis of the glycosylphosphatidylinositol (GPI) anchor, a glycolipid that links dozens of cell-surface proteins to the plasma membrane on hematopoietic cells, including blood group antigens, adhesion molecules and complement regulatory proteins.1,9,10
The mechanism of the PIGA mutation is unknown, but the close association between PNH and acquired aplastic anemia (AA) suggests that the immune attack on hematopoietic stem cells provides a conditional survival advantage to the PNH clone.
Lack of the complement inhibitor CD59 on the RBCs surface is mostly responsible for the clinical manifestations in PNH. These patients manifest with chronic intravascular hemolysis, paroxysmal flares of hemolysis and a propensity for thrombosis.1,9–11 Intravascular hemolysis leads to release of free hemoglobin (Hb) into the blood. Free hemoglobin, in turn, can cause various toxic effects, including hypercoagulability, changes in vascular tone from reduction of circulating nitric oxide and renal damage.
Extravascular hemolysis also occurs in patients with PNH because C3 fragments that are not destroyed by the membrane attack complex (MAC) intravascularly can accumulate on the GPI-negative red blood cell (lacking CD55) surface and these fragments opsonize the RBCs, causing reticuloendothelial destruction in the liver and spleen.1,9–11
DiagnosisLaboratory findings in PNH include typical findings of non-antibody-mediated (Coombs-negative) intravascular hemolysis (anemia, increased reticulocyte count, lactate dehydrogenase [LDH] and bilirubin; decreased haptoglobin, free serum Hb with pink/red serum, hemoglobinuria with pink/red urine, positive dipstick for heme and negative sediment for red blood cells, negative direct antiglobulin [Coombs] test [DAT], loss of GPI-anchored proteins and findings associated with organ damage from hemolysis and/or thrombosis.
Flow cytometry is the most useful and accepted method to confirm the diagnosis of PNH. Some clinicians also use annual flow cytometry to screen patients with an underlying bone marrow disorder (e.g., AA, myelodysplastic syndrome [MDS]) for the development of subclinical PNH. Flow cytometry is performed by incubating the patient’s peripheral blood cells with fluorescently-labeled monoclonal antibodies that bind to GPI-anchored proteins, which are reduced or absent on ≥2 blood cells lineages (preferably granulocytes and monocytes) in PNH.1 GPI-linked proteins that can be assayed include the CD59 and CD55, as well as the CD14, CD15, CD16, CD24, CD45, CD64 and FLuorescent AERolysin (FLAER), a reagent derived from the bacterial toxin aerolysin, which binds directly to the GPI anchor; proaerolysin is the inactive precursor of aerolysin, which also binds to GPI.1,12–18
Differencial diagnosisThe main clinical situations or diseases that should be considered in the differential diagnosis of PNH are:
- •
Coombs-negative hemolytic anemia (e.g., hemoglobinopathies, hereditary spherocytosis), microangiopathic hemolytic anemias, drug- or toxin-induced hemolysis/anemias, disseminated intravascular coagulation and autoimmune hemolysis
- •
Venous thrombosis in atypical sites, including myeloproliferative disorders; solid tumors associated with hypercoagulability; extrinsic compression of vessels, and; inherited/acquired thrombophilias
- •
Anemia and/or other cytopenias related to bone marrow failure syndrome (e.g., aplastic anemia, MDS)
PNH is classified into three different categories18 (Table 1).
Classification of PNH.1,2,18
| (1) Classic PNH |
| PNH with clinical and laboratory findings of intravascular hemolysis without any evidence of bone marrow deficiency. |
| (2) PNH in the setting of another specified bone marrow disorder. |
| Evidence of hemolysis, as well as another specified bone marrow disorder (e.g., aplastic anemia, MDS). |
| (3) Subclinical PNH. |
| Patients with a small population of PNH cells and no clinical or laboratory evidence of hemolysis or thrombosis. This is more commonly detected in patients with another bone marrow disorder. |
Although chronic hemolytic anemia is a common manifestation, nocturnal hemoglobinuria is rare. The severity of anemia seen in PNH varies in from minimal to very severe. Symptoms related to episodes of hemolysis include fatigue, back and abdominal pain, headache and fever. Increase of hemolysis may happen with acute occurrences, such as infections, surgery or transfusions. Several symptoms in PNH are linked to the property of free plasma hemoglobin to scavenge nitric oxide. These include esophageal spasm, priapism, renal insufficiency, thrombosis and pulmonary hypertension. In general, the severity of clinical findings is thought to correlate with the size(s) of the PNH clone(s) in untreated patients; however, some patients may be relatively asymptomatic with a large PNH clone. The main clinical manifestations of PNH are demonstrated in Table 2.1,20–30
Main clinical manifestations of PNH.1,20–30
| Hemolysis |
| Anemia in PNH is often multifactorial and may result from a combination of RBC destruction and lack of production due to underlying bone marrow failure, iron deficiency or other bone marrow insults. The main consequences of hemolysis are: |
| • Dysphagia, abdominal pain, or erectile dysfunction: intravascular hemolysis releases free hemoglobin into the circulation and the resulting depletion of NO may cause symptoms of increased smooth muscle tone. |
| • Pulmonary hypertension: may result from NO depletion in the pulmonary circulation and/or from pulmonary emboli; nevertheless, clinically significant pulmonary hypertension is rare in PNH and when it does occur, it is often associated with pulmonary emboli. |
| • Renal Failure: intravascular hemolysis in PNH can lead to severe acute hemolytic episodes, which can cause acute renal failure from direct toxicity of free heme in the kidney or chronic hemolysis may lead to renal iron deposition (renal hemosiderosis), which can interfere with proximal tubule function and cause interstitial scarring and cortical infarcts. |
| Thrombosis |
| PNH is characterized by thrombosis in atypical locations. Venous thrombosis (intraabdominal vessels, e.g., hepatic vein [Budd-Chiari syndrome] is significantly more common than arterial thrombosis (e.g., cerebral, coronary). |
| The role of hemolysis in promoting thrombosis is suggested by the observation that complement inhibition reduces both hemolysis and thrombotic complications. In the absence of terminal complement inhibition, the risk of thrombosis correlates with the size of the PNH clone; however, it is not known whether this merely reflects the degree of hemolysis or other factors. |
| Specific contributing factors to the hypercoagulability in PNH may include: free Hb released from RBCs scavenges NO, which in turn leads to vasoconstriction and possibly endothelial cell activation and expression of the tissue factor; procoagulant microparticles may be released from platelets undergoing complement-mediated attack; deficiency of proteins involved in fibrinolysis and increases in proinflammatory/prothrombotic cytokine levels. |
| Thrombosis accounts for 40–67% of deaths in patients with PNH. The first thrombotic event (TE) can be fatal and increases risk for death 5- to 10-fold. |
The treatment options for PNH are supportive care, allogeneic hematopoietic stem cell transplantation (HCT) and a complement blockade by the anti-C5 monoclonal antibody eculizumab.1,13,18,19,28,31
Supportive care- •
Oral iron may be required to replace the large urinary losses. Folate and vitamin B12 supplementation are usually recommended.
- •
RBCs transfusion may be necessary when these measures fail to maintain adequate hemoglobin levels.
- •
As bacterial infections can cause an exacerbation of hemolytic crises in patients with PNH, its treatment with antibiotics should be as early as possible.
- •
Glucocorticoids, although widely used, are considered as an empirical therapy (no randomized studies and no clear benefits). In the meantime, under specific situations and over only several days, therapy with steroids might be used to reduce the severity and duration of the hemolytic crises.
- •
In patients without eculizumab and with high PNH clone size (granulocyte clone > 50%), high level of D dimer, pregnancy, perioperative condition and other associated thrombophilic risk factors, without known contraindication to anticoagulation and platelet count stable (>100 × 109/L), primary prophylaxis should be given. Secondary prevention with eculizumab is appropriate in patients who have already had a thromboembolic event related to PNH.
- •
Immunosuppressive treatment should be considered in PNH/AA and bone marrow deficiency. It is not indicated to treat hemolysis crises or activity.
Although a curative treatment, allogeneic HCT for PNH is generally limited to the most severely affected patients because of significant morbidities and mortality.32–34 Candidates for HCT generally include those with life-threatening disease: severe AA who have an available HLA-matched donor; some high-risk MDS; PNH complications unresponsive to eculizumab or unavailable eculizumab. This decision should be discussed and shared with each possible candidate, with the input from a clinician with expertise in managing PNH.1,32–35
EculizumabEculizumab (Soliris®) is a humanized, first-in-class, anti-C5 antibody and the only approved for PNH by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA), as well as by ANVISA in Brazil in March, 2017.1 Soliris binds with high affinity to C5 and blocks the terminal complement - C5a and C5b-9 formation, reducing the chronic uncontrolled complement activation and its consequences.1,13,36–41 Evidence for the efficacy and safety of eculizumab in PNH comes from a randomized trial and several observational studies.36–42 Eculizumab treatment was associated with the following improved outcomes, compared to placebo: improved survival with a well-tolerated safety profile, persistent and significant improvement in symptoms and quality of life and a significant reduction in hemolysis and number of thrombotic events, as well as in transfusion.1,13,19,36–42
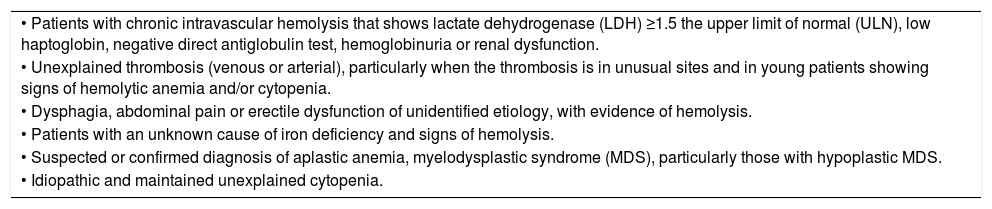
Indications for PNH screeningThe indications for PNH screening should be performed in patients with one or more of the symptoms listed in Table 3.
Indications for PNH screening.1,13,19,28,31
| • Patients with chronic intravascular hemolysis that shows lactate dehydrogenase (LDH) ≥1.5 the upper limit of normal (ULN), low haptoglobin, negative direct antiglobulin test, hemoglobinuria or renal dysfunction. |
| • Unexplained thrombosis (venous or arterial), particularly when the thrombosis is in unusual sites and in young patients showing signs of hemolytic anemia and/or cytopenia. |
| • Dysphagia, abdominal pain or erectile dysfunction of unidentified etiology, with evidence of hemolysis. |
| • Patients with an unknown cause of iron deficiency and signs of hemolysis. |
| • Suspected or confirmed diagnosis of aplastic anemia, myelodysplastic syndrome (MDS), particularly those with hypoplastic MDS. |
| • Idiopathic and maintained unexplained cytopenia. |
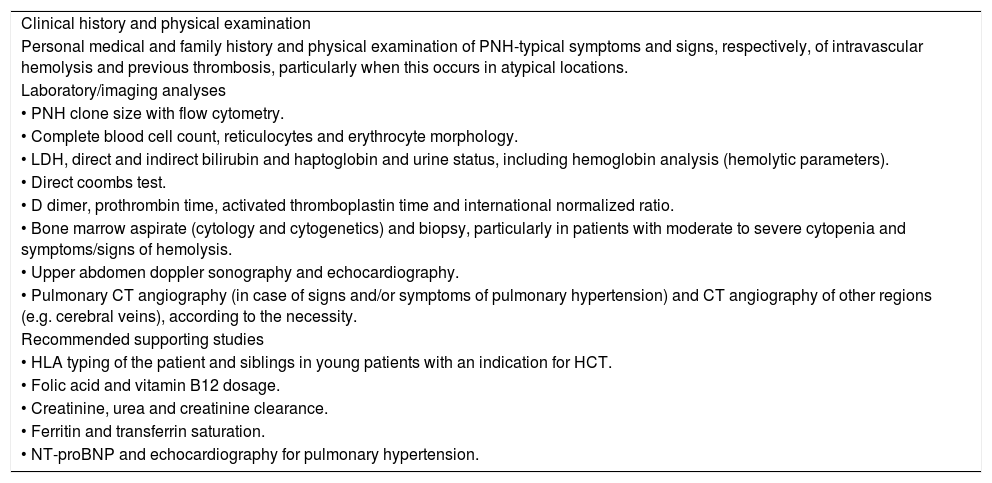
The main clinical and laboratory evaluation for patients whose diagnosis of PNH has been confirmed are listed in Table 4.
Clinical and laboratory evaluation of PNH patients.1,13,18,19,28,31
| Clinical history and physical examination |
| Personal medical and family history and physical examination of PNH-typical symptoms and signs, respectively, of intravascular hemolysis and previous thrombosis, particularly when this occurs in atypical locations. |
| Laboratory/imaging analyses |
| • PNH clone size with flow cytometry. |
| • Complete blood cell count, reticulocytes and erythrocyte morphology. |
| • LDH, direct and indirect bilirubin and haptoglobin and urine status, including hemoglobin analysis (hemolytic parameters). |
| • Direct coombs test. |
| • D dimer, prothrombin time, activated thromboplastin time and international normalized ratio. |
| • Bone marrow aspirate (cytology and cytogenetics) and biopsy, particularly in patients with moderate to severe cytopenia and symptoms/signs of hemolysis. |
| • Upper abdomen doppler sonography and echocardiography. |
| • Pulmonary CT angiography (in case of signs and/or symptoms of pulmonary hypertension) and CT angiography of other regions (e.g. cerebral veins), according to the necessity. |
| Recommended supporting studies |
| • HLA typing of the patient and siblings in young patients with an indication for HCT. |
| • Folic acid and vitamin B12 dosage. |
| • Creatinine, urea and creatinine clearance. |
| • Ferritin and transferrin saturation. |
| • NT-proBNP and echocardiography for pulmonary hypertension. |
The main inclusion criteria for eculizumab treatment are: patients with hemolysis (LDH ≥ 1.5 ULN), symptomatic, and any of the following criteria:
- •
Hb < 7 g/dL or Hb < 10 g/dL, in at least two independent measurements in a patient with cardiac symptoms.
- •
Thrombosis related to PNH.
- •
Complications associated with hemolysis: renal dysfunction and pulmonary hypertension.
- •
Abdominal pain and/or dysphagia and/or erectile dysfunction.
- •
Pregnancy, particularly those with a previous history of gestational complications.
It is important to note that the presence of these inclusion criteria are sufficient to consider treatment with eculizumab, regardless of the need for transfusions, as well as the PNH clone size (although knowing that patients with large PNH clone size [>50%] are more likely to present relevant intravascular hemolysis).
Management of PNHIt is important to classify PNH according to the flow cytometry results for reticulocyte count, serum LDH concentration and bone marrow analysis:
- •
Subclinical PNH: no specific PNH therapy. Attention to bone marrow failure (BMF) syndrome.
- •
PNH/BMF syndrome: focus on BMF. Patients who present very important PNH clones may benefit from eculizumab.
- •
Classic PNH: eculizumab.
Eculizumab should not be considered for patients with any of the following criteria:1,13,19,28,31
- •
Mild or no symptoms attributable to hemolysis.
- •
Small granulocyte clone size (<30%), without evidence of hemolysis and with normal blood counts.
- •
Eculizumab does not modify the underlying hematopoietic stem cell defect responsible for PNH. Thus, it is not curative and has not been proven to be beneficial in patients with AA or MDS, without hemolysis or thrombosis. Eculizumab is not used in this setting due to the high cost and importance of other therapies directed at the underlying AA or MDS.
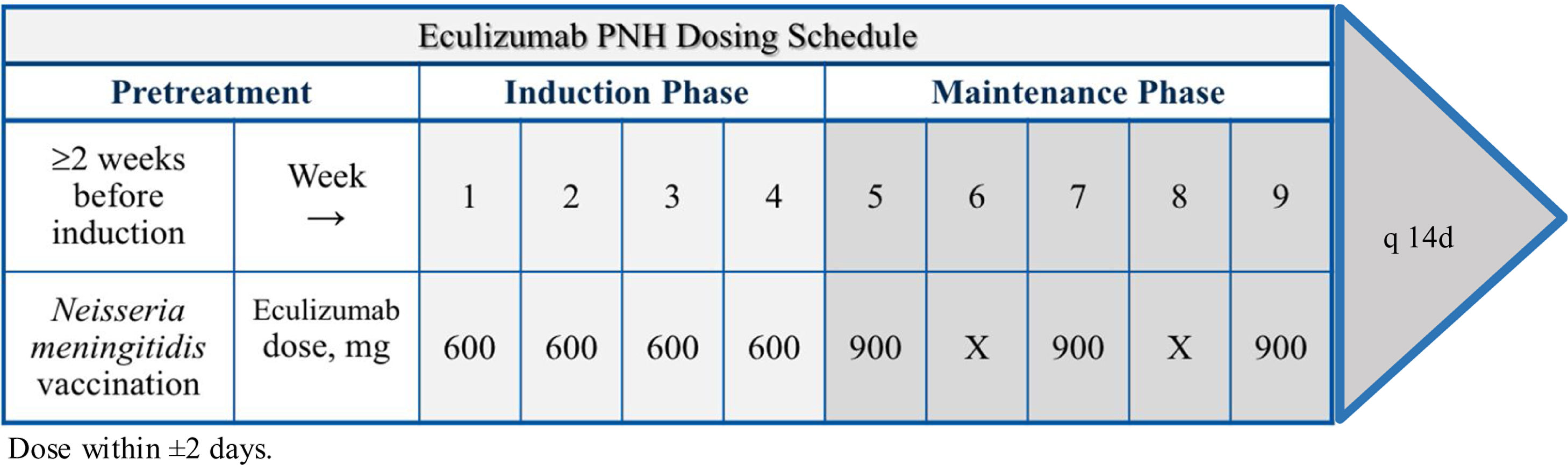
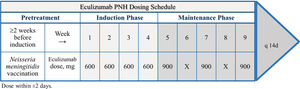
Eculizumab is administered intravenously within 25–45 min every 7 days during induction and every 14 days during maintenance, as demonstrated in Figure 1.19,28 The shortening of the time interval (to 12 days) or increasing the dose of eculizumab (to 1200 mg) should be considered if there are signs of worsening hemolysis.19,28,31
Risks and problems with eculizumabInfectionEculizumab increases the risk of life-threatening Neisseria infections, including N. meningitidis. 1 A tetravalent vaccine against serotypes ACYW135 and serogroup B is strongly recommended to be administered to patients at least two weeks prior to receiving the first dose of eculizumab.1,36,19,31,36 Children at less than 2 years of age, or without vaccination at least 14 days before the beginning of the treatment with eculizumab, must receive treatment with appropriate prophylactic antibiotics until 14 days after vaccination. Revaccination according to current medical guidelines for vaccine use is usually recommended every 2.5–3 years. Vaccination is sometimes not sufficient to prevent meningococcal infection and the use of antibacterial agents needs to be taken in consideration. Patients should be observed for signs of meningococcal infection, action should be taken immediately if infection is suspected and antibiotics should be administered, if necessary.36
For patients who must receive eculizumab emergently (e.g., severe hemolytic crisis or acute onset thrombosis), prophylactic antibiotics with ciprofloxacin to prevent meningitis may be administered concurrently with eculizumab for two to three weeks until the vaccinations take effect. Despite not having been formally studied, all patients on eculizumab should receive penicillin prophylaxis.
In suspected cases of meningococcal infection, it is mandatory that the patient be instructed to start empirical treatment with 750 mg of ciprofloxacin, as well as to seek emergency services as soon as possible to confirm or refute the diagnosis.19,31
Elective surgical proceduresIn patients scheduled for elective surgery, the procedure should be done the day after the last administration of eculizumab. Surgical procedures can activate the complement cascade, with terminal complex formation and therefore, in case of breakthrough hemolysis, an extra dose of eculizumab should be considered to control the hemolysis. In addition, perioperative prophylaxis with low-molecular-weight heparin is mandatory.1,19,31
Hemoglobin variation and extravascular hemolysisMore than two-thirds of patients will respond well to eculizumab. However, variations in Hb values can be observed due to several factors, such as: associated inflammatory process, percentage of the PNH clone, degree of bone marrow failure and genetic factors.1,40
Extravascular hemolysis can persist in individuals treated with eculizumab because C3 fragments can accumulate on RBCs that are not destroyed by the MAC intravascularly and these fragments opsonize the RBCs. Thus, patients receiving eculizumab may develop extravascular hemolysis that may reduce the clinical benefits of the drug. Usually, this condition is characterized by mild to moderate anemia and a positive direct antiglobulin (Coombs) test (DAT) for C3d. This is generally not severe enough to necessitate transfusions, although about one-fourth of patients remain transfusion-dependent.1,11,40
Polymorphisms in the complement receptor 1 and response to eculizumabGenetic polymorphisms may also affect response.1,44,45 A study on 345 Japanese patients with PNH found that hemolysis did not improve with eculizumab in 11 (3.2 percent); these patients were heterozygous for a single missense mutation in the C5 that prevents eculizumab binding and is present in 3.5 percent of the normal Japanese population.45 A polymorphism in the complement receptor 1 (CR1) gene, which regulates the binding of C3 to RBCs, was also associated with reduced efficacy of eculizumab.1,44,45
Next-generation anti-complement drugsA variety of other approaches to blocking complement activation, including monoclonal antibodies and other anti-complement proteins (peptide inhibitors, small molecule inhibitors and decoy receptors), are also in various stages of preclinical/clinical development.46–48 Similar to eculizumab, in terms of efficacy and safety, ravulizumab is a new monoclonal antibody which binds to C5 to prevent cleavage of C5 by C5 convertases and has the advantage that, by presenting a longer half-life, it can be administered every eight weeks.46
Pregnancy and PNHPregnancy is not contraindicated for PNH patients, but all female PNH patients who desire to become pregnant should be informed about careful risk/benefit and all their doubts clarified on the increased risks of morbidity (particularly thrombosis) and mortality for the mother, as well as for the fetus.49 Treatment with eculizumab may prevent maternal and fetal complications and therefore, pregnant patients on eculizumab should not discontinue the drug during the pregnancy and breastfeeding.1,49,50 Preventive prophylaxis with warfarin might be used.1,13,19,31,49,50
Monitoring the PNH patient on eculizumab1,13,19,28,31The main parameters of monitoring the treatment with eculizumab should be performed regularly and include:
- •
Complete blood count with differential, reticulocyte count, LDH and biochemical: monthly in the first 3 months, then every 3 months.
- •
Renal function (urea, electrolytes and eGFI): every 3 months.
- •
Iron studies (transferrin saturation and ferritin): every 3 months.
- •
Information of transfusion history: every 6 months.
- •
Folic acid and vitamin B12 dosage: yearly or before, if there is evidence of folic acid/vitamin B12 deficiency.
- •
PNH clone analysis every 6 months over the first two years and thereafter, once a year for those under eculizumab treatment and with stable disease.
Eculizumab therapy prospects1,13,19,28,31
The results of treatment with eculizumab should be reported every 3–6 months, following the patient’s clinical and laboratorial response. The most important results expected with eculizumab treatment are:
In 1 week:
- •
Decrease in hemolysis (as measured by LDH).
- •
Decrease in fatigue.
Between 2 and 3 weeks:
- •
Amelioration in quality of life.
- •
Amelioration in dyspnea.
Between 2 and 6 months:
- •
Reduction in the number of transfusions.
- •
No variation of hemoglobin levels.
>6 months:
- •
Continued improvement in quality of life.
- •
Normal LDH levels.
- •
Continued improvement in fatigue.
- •
Continued reduction in the number of transfusions.
- •
Stable hemoglobin levels.
At 36 months and up to 10 years:
- •
Maintained decrease in hemolysis (as measured by LDH).
- •
Continued improvement in thrombotic events.
- •
Commonly described collateral events with mild or moderate severity.
Long-term use of eculizumab demonstrates the impact on the quality of life and reduction in the thrombotic event rate and other complications, thereby improving long-term outcomes for patients with PNH.
Treatment discontinuation should be discussed in the context of the absence of clinical benefit and one or more of associated clinical or laboratorial features:
- •
RBC transfusion requirement reduction of less than 30% 6 months after the first eculizumab dose administration.
- •
Continuity of significant hemolysis in the context of the absence or minimal basal Hb level improvement after the first eculizumab dose administration.
- •
Spontaneous disease remission.
- •
Patients who develop severe bone marrow failure syndrome.
- •
Patient’s decision to stop treatment or lack of patient adherence to treatment and clinical and laboratory control procedures.
Although eculizumab has robust technical data in the literature to substantiate its benefit in PNH, the main limitation in access to medication is its high cost. Given the limited context of funding available to the Brazilian public health system (SUS), indiscriminate drug indications could have serious consequences for the public budget, making it impossible to adopt other expensive public policies in a resource-poor country.
For this reason, the ABHH RBC and Iron Committee (Comitê de Glóbulos Vermelhos e do Ferro da ABHH) has taken the initiative of this consensus statement for the diagnosis and treatment of PNH to broadly, comprehensively and responsibly benefit patients with PNH who present severity criteria for specific treatment.
Conflicts of interestThe authors declare no conflicts of interest.