Individuals with sickle cell anemia may suffer symptomatic or silent cerebral infarcts leading to neurocognitive complications. This study investigated the cognitive and intellectual performance of children and adolescents with sickle cell anemia.
MethodsThe socioeconomic status, clinical aspects and behavioral profile of 15 young individuals with sickle cell anemia were evaluated. The Wechsler Intelligence Scale for Children, the Developmental Neuropsychological Assessment Test, and the Child Behavior Checklist were applied.
ResultsParticipants with a history of stroke had lower intelligence quotient (IQ) scores. Alterations were found in attention and executive functioning, language, verbal and visual memory, visuospatial processing and sensorimotor skills. These alterations were found both in the children and adolescents who had had a cerebral infarction and in those who apparently had not. In the majority of cases, there were learning difficulties, a history of repeating school years and a need for specialist educational support. The most common additional diagnoses in accordance with the Diagnostic and Statistical Manual of Mental Disorders IV were depressive disorder, anxiety disorder and somatic disorder, as well as conditions associated with physical and psychosocial repercussions of sickle cell anemia.
ConclusionAs sickle cell anemia is considered a progressive cerebral vasculopathy, it is a potential risk factor for neurocognitive and psychosocial development. Therefore, periodic neuropsychological and behavioral evaluations of children and adolescents with sickle cell anemia may represent a useful measure to reduce long-term biopsychosocial repercussions.
Sickle cell disease (SCD) comprises a group of inherited blood disorders resulting from a genetic alteration in hemoglobin. It is the most common monogenic hereditary disease in Brazil.1 In the state of Bahia, the number of individuals with the sickle cell trait is estimated at 5.5% of the general population and 6.3% of the population of African descent. The incidence of sickle cell anemia (SCA) in Bahia is the highest in Brazil: 1 in 650 live births.2
Ohene-Frempong et al. reported that 11% of individuals with SCA develop symptomatic cerebral infarcts prior to completing 20 years of age. The sequelae of these events affect different aspects of the patient's life. Overall, 11–35% of children and adolescents with SCD present with silent strokes identified only by imaging exams and with no observable clinical symptoms.3
The neurocognitive complications and their consequences are progressive and originate from a state of cerebral vasculopathy, negatively affecting child development and quality of life.4
The principal objective of this study was to evaluate cognitive function and the behavioral profile of children and adolescents with sickle cell anemia.
MethodsThe Internal Review Board of the Plataforma Brasil of the Professor Edgard Santos Teaching Hospital, Universidade Federal da Bahia approved the protocol of this descriptive, observational, hospital-based study (#314.636).
Fifteen children and adolescents with ages ranging from 6 to 16 years and SCA diagnosed by hemoglobin electrophoresis participated in this study. Children with visual or auditory deficits, epilepsy, a history of brain trauma, meningitis or intoxications, a history of gestational or neonatal anoxic brain injury, prematurity, concomitant genetic syndromes or any other condition that would prevent the psychometric tests from being conducted were excluded from the study.
The children's parents or guardians were interviewed at admission. The child's birth registration card was reviewed to obtain data related to pregnancy and neonatal conditions. The school report was used to evaluate neuropsychomotor development and educational history, data on the clinical characteristics of SCD were obtained and the socioeconomic criteria of the Associação Brasileira de Empresas de Pesquisa (ABEP) were applied.5
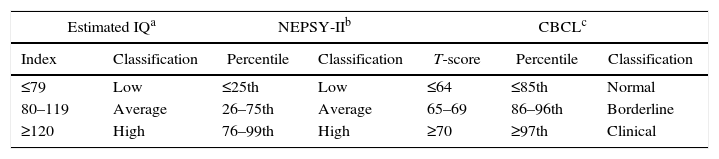
A short form of the Wechsler Intelligence Scale for Children (Third Edition – WISC-III) was used to estimate the intelligence quotient (IQ). This instrument is used as a screening tool to evaluate the intellectual performance of Brazilian children (Table 1).6,7
Classification of performance according to the Wechsler Intelligence Scale for Children (Third Edition) intelligence quotient (IQ), Developmental Neuropsychological Assessment Test (second edition) and Child Behavior Checklist.
Fifteen subtests of the developmental neuropsychological assessment test (second edition – NEPSY-II)8 were selected to investigate cognitive function. This tool takes a broad and flexible approach to neuropsychological evaluation. Percentiles were used to classify performance in this assessment (Table 1).
The Child Behavior Checklist (CBCL), a questionnaire consisting of 113 items, developed to evaluate individuals in the 6–18 age range, was applied to parents or guardians9 (Table 1).
ResultsOf the fifteen cases evaluated, nine (60%) were male. The mean age of the patients was nine years (range: 6–16 years). Of the 15 patients, 12 (80%) were right-handed, while three (20%) became left-handed after having suffered a left hemisphere stroke. In these cases, the trained left hand was considered the dominant hand.
In nine (60%) cases, the family reported that the child needed specialist educational support due to difficulties at school.
The IQ test was found to be within the normal range for ten (66.6%) participants, while four (26.6%) had a lower than average score, and one (6.6%) scored above average. Of the four (26.6%) participants who scored below average, three (20%) had a borderline score (70–79). The mean IQ score was 88.5.
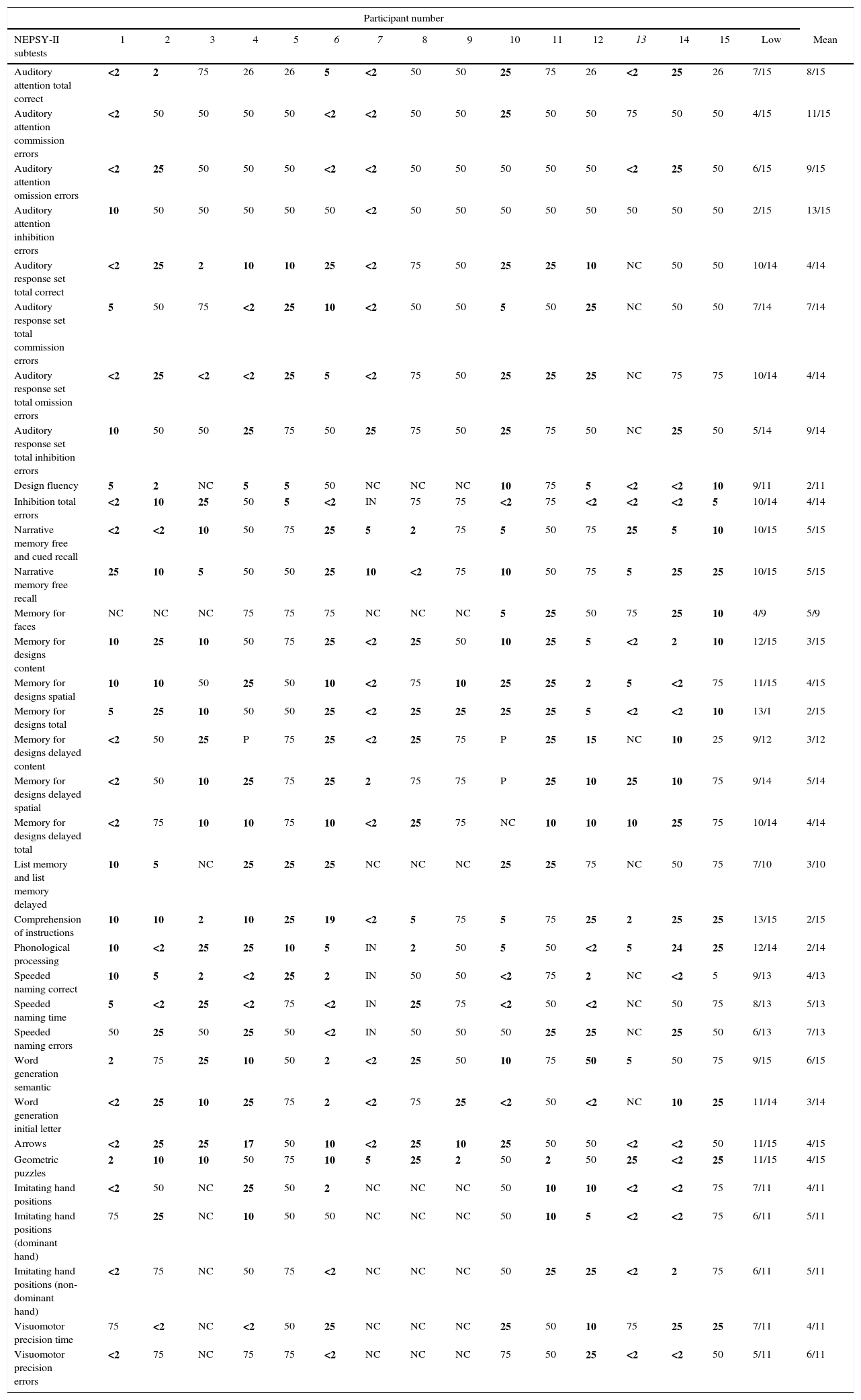
In the majority of cases, the individual's performance with respect to immediate memory was poor for visual, spatial and verbal content as well as in the subtests of attention and executive functioning, language and sensorimotor skills (Table 2). Overall, nine (60%) participants performed below average in at least one of the NEPSY-II subtests (Table 3).
Performance in the Developmental Neuropsychological Assessment Test (second edition) subtests.
| Participant number | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NEPSY-II subtests | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Low | Mean |
| Auditory attention total correct | <2 | 2 | 75 | 26 | 26 | 5 | <2 | 50 | 50 | 25 | 75 | 26 | <2 | 25 | 26 | 7/15 | 8/15 |
| Auditory attention commission errors | <2 | 50 | 50 | 50 | 50 | <2 | <2 | 50 | 50 | 25 | 50 | 50 | 75 | 50 | 50 | 4/15 | 11/15 |
| Auditory attention omission errors | <2 | 25 | 50 | 50 | 50 | <2 | <2 | 50 | 50 | 50 | 50 | 50 | <2 | 25 | 50 | 6/15 | 9/15 |
| Auditory attention inhibition errors | 10 | 50 | 50 | 50 | 50 | 50 | <2 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 2/15 | 13/15 |
| Auditory response set total correct | <2 | 25 | 2 | 10 | 10 | 25 | <2 | 75 | 50 | 25 | 25 | 10 | NC | 50 | 50 | 10/14 | 4/14 |
| Auditory response set total commission errors | 5 | 50 | 75 | <2 | 25 | 10 | <2 | 50 | 50 | 5 | 50 | 25 | NC | 50 | 50 | 7/14 | 7/14 |
| Auditory response set total omission errors | <2 | 25 | <2 | <2 | 25 | 5 | <2 | 75 | 50 | 25 | 25 | 25 | NC | 75 | 75 | 10/14 | 4/14 |
| Auditory response set total inhibition errors | 10 | 50 | 50 | 25 | 75 | 50 | 25 | 75 | 50 | 25 | 75 | 50 | NC | 25 | 50 | 5/14 | 9/14 |
| Design fluency | 5 | 2 | NC | 5 | 5 | 50 | NC | NC | NC | 10 | 75 | 5 | <2 | <2 | 10 | 9/11 | 2/11 |
| Inhibition total errors | <2 | 10 | 25 | 50 | 5 | <2 | IN | 75 | 75 | <2 | 75 | <2 | <2 | <2 | 5 | 10/14 | 4/14 |
| Narrative memory free and cued recall | <2 | <2 | 10 | 50 | 75 | 25 | 5 | 2 | 75 | 5 | 50 | 75 | 25 | 5 | 10 | 10/15 | 5/15 |
| Narrative memory free recall | 25 | 10 | 5 | 50 | 50 | 25 | 10 | <2 | 75 | 10 | 50 | 75 | 5 | 25 | 25 | 10/15 | 5/15 |
| Memory for faces | NC | NC | NC | 75 | 75 | 75 | NC | NC | NC | 5 | 25 | 50 | 75 | 25 | 10 | 4/9 | 5/9 |
| Memory for designs content | 10 | 25 | 10 | 50 | 75 | 25 | <2 | 25 | 50 | 10 | 25 | 5 | <2 | 2 | 10 | 12/15 | 3/15 |
| Memory for designs spatial | 10 | 10 | 50 | 25 | 50 | 10 | <2 | 75 | 10 | 25 | 25 | 2 | 5 | <2 | 75 | 11/15 | 4/15 |
| Memory for designs total | 5 | 25 | 10 | 50 | 50 | 25 | <2 | 25 | 25 | 25 | 25 | 5 | <2 | <2 | 10 | 13/1 | 2/15 |
| Memory for designs delayed content | <2 | 50 | 25 | P | 75 | 25 | <2 | 25 | 75 | P | 25 | 15 | NC | 10 | 25 | 9/12 | 3/12 |
| Memory for designs delayed spatial | <2 | 50 | 10 | 25 | 75 | 25 | 2 | 75 | 75 | P | 25 | 10 | 25 | 10 | 75 | 9/14 | 5/14 |
| Memory for designs delayed total | <2 | 75 | 10 | 10 | 75 | 10 | <2 | 25 | 75 | NC | 10 | 10 | 10 | 25 | 75 | 10/14 | 4/14 |
| List memory and list memory delayed | 10 | 5 | NC | 25 | 25 | 25 | NC | NC | NC | 25 | 25 | 75 | NC | 50 | 75 | 7/10 | 3/10 |
| Comprehension of instructions | 10 | 10 | 2 | 10 | 25 | 19 | <2 | 5 | 75 | 5 | 75 | 25 | 2 | 25 | 25 | 13/15 | 2/15 |
| Phonological processing | 10 | <2 | 25 | 25 | 10 | 5 | IN | 2 | 50 | 5 | 50 | <2 | 5 | 24 | 25 | 12/14 | 2/14 |
| Speeded naming correct | 10 | 5 | 2 | <2 | 25 | 2 | IN | 50 | 50 | <2 | 75 | 2 | NC | <2 | 5 | 9/13 | 4/13 |
| Speeded naming time | 5 | <2 | 25 | <2 | 75 | <2 | IN | 25 | 75 | <2 | 50 | <2 | NC | 50 | 75 | 8/13 | 5/13 |
| Speeded naming errors | 50 | 25 | 50 | 25 | 50 | <2 | IN | 50 | 50 | 50 | 25 | 25 | NC | 25 | 50 | 6/13 | 7/13 |
| Word generation semantic | 2 | 75 | 25 | 10 | 50 | 2 | <2 | 25 | 50 | 10 | 75 | 50 | 5 | 50 | 75 | 9/15 | 6/15 |
| Word generation initial letter | <2 | 25 | 10 | 25 | 75 | 2 | <2 | 75 | 25 | <2 | 50 | <2 | NC | 10 | 25 | 11/14 | 3/14 |
| Arrows | <2 | 25 | 25 | 17 | 50 | 10 | <2 | 25 | 10 | 25 | 50 | 50 | <2 | <2 | 50 | 11/15 | 4/15 |
| Geometric puzzles | 2 | 10 | 10 | 50 | 75 | 10 | 5 | 25 | 2 | 50 | 2 | 50 | 25 | <2 | 25 | 11/15 | 4/15 |
| Imitating hand positions | <2 | 50 | NC | 25 | 50 | 2 | NC | NC | NC | 50 | 10 | 10 | <2 | <2 | 75 | 7/11 | 4/11 |
| Imitating hand positions (dominant hand) | 75 | 25 | NC | 10 | 50 | 50 | NC | NC | NC | 50 | 10 | 5 | <2 | <2 | 75 | 6/11 | 5/11 |
| Imitating hand positions (non-dominant hand) | <2 | 75 | NC | 50 | 75 | <2 | NC | NC | NC | 50 | 25 | 25 | <2 | 2 | 75 | 6/11 | 5/11 |
| Visuomotor precision time | 75 | <2 | NC | <2 | 50 | 25 | NC | NC | NC | 25 | 50 | 10 | 75 | 25 | 25 | 7/11 | 4/11 |
| Visuomotor precision errors | <2 | 75 | NC | 75 | 75 | <2 | NC | NC | NC | 75 | 50 | 25 | <2 | <2 | 50 | 5/11 | 6/11 |
NC: test not carried out for age; IN: inability to answer test.
Scores compared to percentiles of the Brazilian sample. Poor results in bold. Forced right-handed individuals in italics.
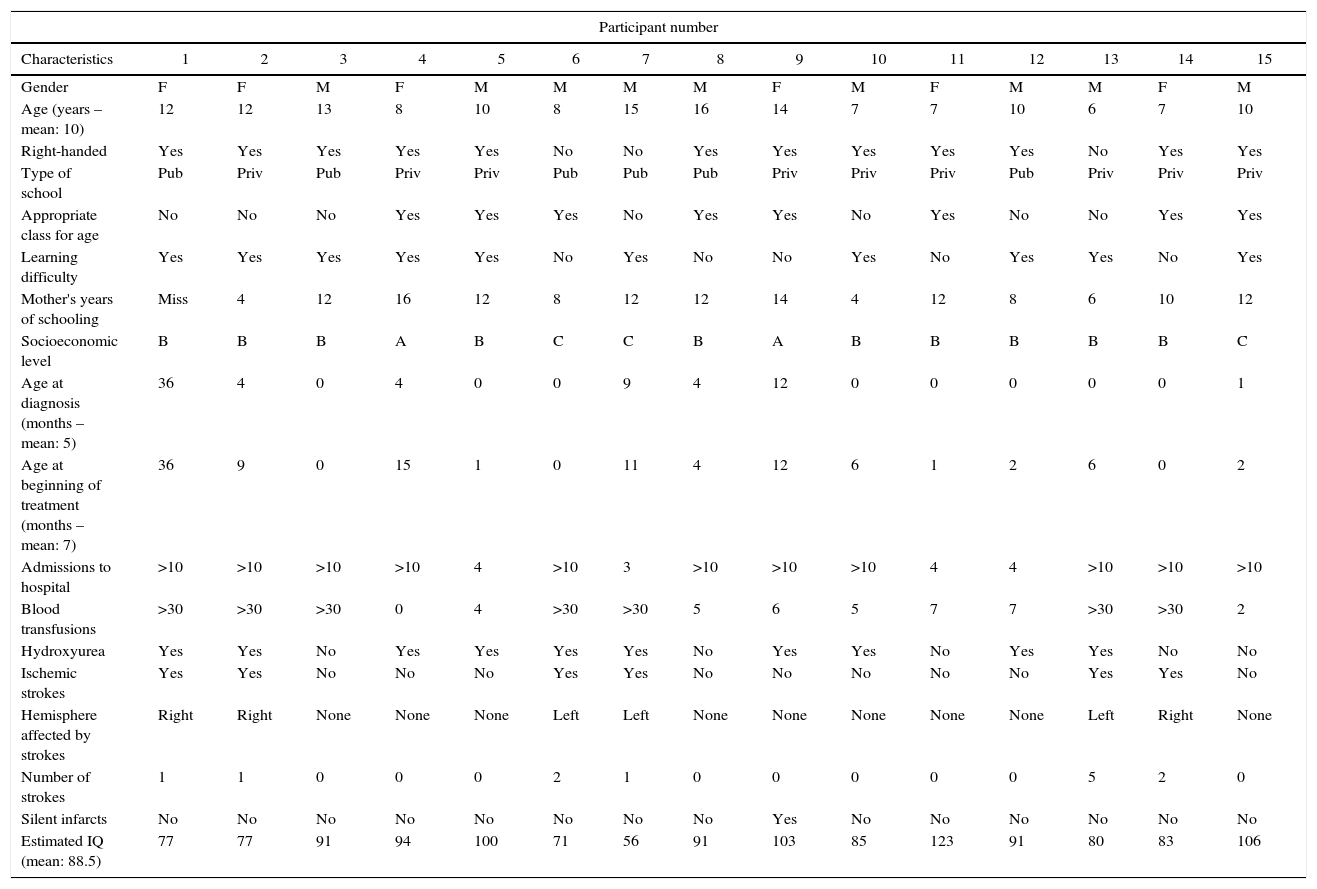
Socioeconomic and clinical classification of the children and adolescents with sickle cell anemia.
| Participant number | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| Gender | F | F | M | F | M | M | M | M | F | M | F | M | M | F | M |
| Age (years – mean: 10) | 12 | 12 | 13 | 8 | 10 | 8 | 15 | 16 | 14 | 7 | 7 | 10 | 6 | 7 | 10 |
| Right-handed | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Type of school | Pub | Priv | Pub | Priv | Priv | Pub | Pub | Pub | Priv | Priv | Priv | Pub | Priv | Priv | Priv |
| Appropriate class for age | No | No | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | No | Yes | Yes |
| Learning difficulty | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes | No | Yes | Yes | No | Yes |
| Mother's years of schooling | Miss | 4 | 12 | 16 | 12 | 8 | 12 | 12 | 14 | 4 | 12 | 8 | 6 | 10 | 12 |
| Socioeconomic level | B | B | B | A | B | C | C | B | A | B | B | B | B | B | C |
| Age at diagnosis (months – mean: 5) | 36 | 4 | 0 | 4 | 0 | 0 | 9 | 4 | 12 | 0 | 0 | 0 | 0 | 0 | 1 |
| Age at beginning of treatment (months – mean: 7) | 36 | 9 | 0 | 15 | 1 | 0 | 11 | 4 | 12 | 6 | 1 | 2 | 6 | 0 | 2 |
| Admissions to hospital | >10 | >10 | >10 | >10 | 4 | >10 | 3 | >10 | >10 | >10 | 4 | 4 | >10 | >10 | >10 |
| Blood transfusions | >30 | >30 | >30 | 0 | 4 | >30 | >30 | 5 | 6 | 5 | 7 | 7 | >30 | >30 | 2 |
| Hydroxyurea | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | No |
| Ischemic strokes | Yes | Yes | No | No | No | Yes | Yes | No | No | No | No | No | Yes | Yes | No |
| Hemisphere affected by strokes | Right | Right | None | None | None | Left | Left | None | None | None | None | None | Left | Right | None |
| Number of strokes | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 5 | 2 | 0 |
| Silent infarcts | No | No | No | No | No | No | No | No | Yes | No | No | No | No | No | No |
| Estimated IQ (mean: 88.5) | 77 | 77 | 91 | 94 | 100 | 71 | 56 | 91 | 103 | 85 | 123 | 91 | 80 | 83 | 106 |
M: male; F: female; Miss: missing data; Pub: public; Priv: private; m: mean (range); f: frequency.
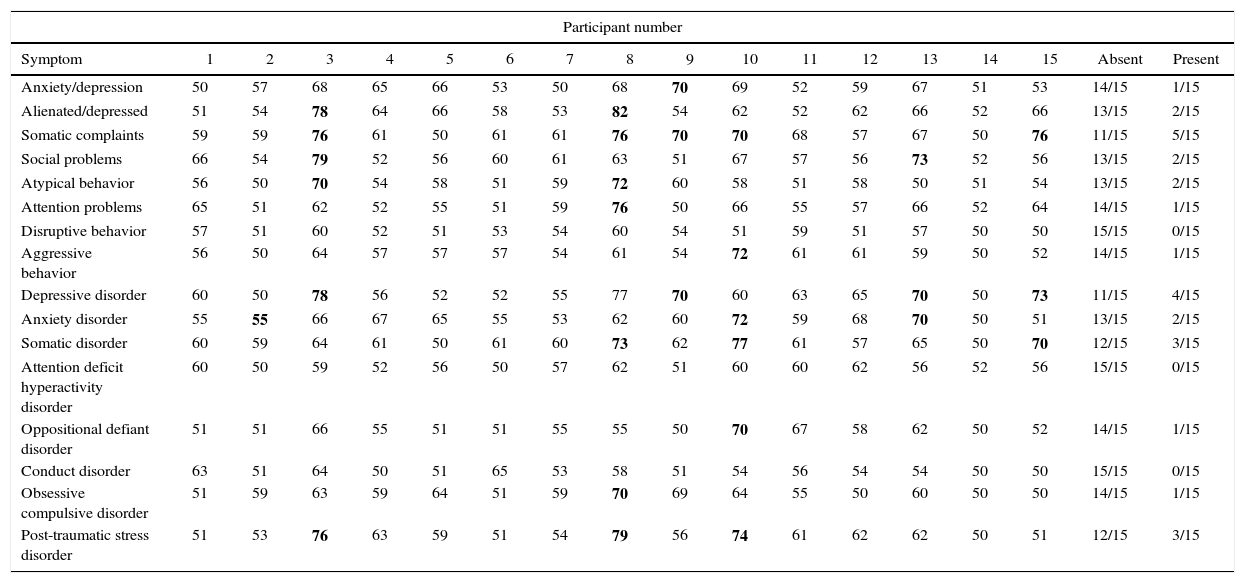
The behavioral profile and diagnostic criteria based on the Diagnostic and Statistical Manual of Mental Disorders (fourth Edition) are described in Table 4.
Behavioral profile and Statistical Manual of Mental Disorders 4th Edition diagnoses from the Child Behavior Checklist evaluation.
| Participant number | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptom | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Absent | Present |
| Anxiety/depression | 50 | 57 | 68 | 65 | 66 | 53 | 50 | 68 | 70 | 69 | 52 | 59 | 67 | 51 | 53 | 14/15 | 1/15 |
| Alienated/depressed | 51 | 54 | 78 | 64 | 66 | 58 | 53 | 82 | 54 | 62 | 52 | 62 | 66 | 52 | 66 | 13/15 | 2/15 |
| Somatic complaints | 59 | 59 | 76 | 61 | 50 | 61 | 61 | 76 | 70 | 70 | 68 | 57 | 67 | 50 | 76 | 11/15 | 5/15 |
| Social problems | 66 | 54 | 79 | 52 | 56 | 60 | 61 | 63 | 51 | 67 | 57 | 56 | 73 | 52 | 56 | 13/15 | 2/15 |
| Atypical behavior | 56 | 50 | 70 | 54 | 58 | 51 | 59 | 72 | 60 | 58 | 51 | 58 | 50 | 51 | 54 | 13/15 | 2/15 |
| Attention problems | 65 | 51 | 62 | 52 | 55 | 51 | 59 | 76 | 50 | 66 | 55 | 57 | 66 | 52 | 64 | 14/15 | 1/15 |
| Disruptive behavior | 57 | 51 | 60 | 52 | 51 | 53 | 54 | 60 | 54 | 51 | 59 | 51 | 57 | 50 | 50 | 15/15 | 0/15 |
| Aggressive behavior | 56 | 50 | 64 | 57 | 57 | 57 | 54 | 61 | 54 | 72 | 61 | 61 | 59 | 50 | 52 | 14/15 | 1/15 |
| Depressive disorder | 60 | 50 | 78 | 56 | 52 | 52 | 55 | 77 | 70 | 60 | 63 | 65 | 70 | 50 | 73 | 11/15 | 4/15 |
| Anxiety disorder | 55 | 55 | 66 | 67 | 65 | 55 | 53 | 62 | 60 | 72 | 59 | 68 | 70 | 50 | 51 | 13/15 | 2/15 |
| Somatic disorder | 60 | 59 | 64 | 61 | 50 | 61 | 60 | 73 | 62 | 77 | 61 | 57 | 65 | 50 | 70 | 12/15 | 3/15 |
| Attention deficit hyperactivity disorder | 60 | 50 | 59 | 52 | 56 | 50 | 57 | 62 | 51 | 60 | 60 | 62 | 56 | 52 | 56 | 15/15 | 0/15 |
| Oppositional defiant disorder | 51 | 51 | 66 | 55 | 51 | 51 | 55 | 55 | 50 | 70 | 67 | 58 | 62 | 50 | 52 | 14/15 | 1/15 |
| Conduct disorder | 63 | 51 | 64 | 50 | 51 | 65 | 53 | 58 | 51 | 54 | 56 | 54 | 54 | 50 | 50 | 15/15 | 0/15 |
| Obsessive compulsive disorder | 51 | 59 | 63 | 59 | 64 | 51 | 59 | 70 | 69 | 64 | 55 | 50 | 60 | 50 | 50 | 14/15 | 1/15 |
| Post-traumatic stress disorder | 51 | 53 | 76 | 63 | 59 | 51 | 54 | 79 | 56 | 74 | 61 | 62 | 62 | 50 | 51 | 12/15 | 3/15 |
Bold: when present. Values described as T-scores.
The participants’ socioeconomic levels were similar. Most of the children studied in private schools near their homes, where the pedagogical methodology used and the requirements are similar to those in public schools.
The common symptoms of SCA were present in all cases and all the participants were undergoing specialist treatment, as required by the Ministry of Health under regulation #55 published in 2010.2 In compliance with a clinical protocol, most of the individuals were taking hydroxyurea10 and were receiving blood transfusions, treatments that are based on clinical and scientific consensus.2,11
Performance in intelligence tests is generally associated with academic achievement and both have proven useful measures for monitoring a child's cognitive development.12 The individuals in this study who had had a stroke had lower IQ scores and were the only participants who scored poorly in the WISC-III subtests of vocabulary and block design. Two of the six individuals with a history of stroke had an IQ score within the normal range; nevertheless, these scores were lower than those obtained by the participants who had not had strokes. Patients who had suffered strokes performed poorly in all the functions evaluated. Indeed, having had a stroke predisposes the individual to more severe alterations in intellectual and cognitive development, as shown in previous studies.13 Although this finding is not surprising, it emphasizes the fact that even one stroke may define a child's life, reducing his/her potential in academic development and reducing employment opportunities in adult life. In childhood, the cost of performing poorly at school is obviously reflected in a reduction of formal learning and a decrease in cognitive reserve. Frequent hospital admissions together with poor school performance constitute the principal causes of school dropout in these children.14
In general, insofar as the neuropsychological evaluation is concerned children with SCA performed poorly. None of the children fit the normal profile of a typical child in any of the tests used in this study. Of the ten children whose IQ was within the normal range, six had learning difficulties. Therefore, even the children with an average IQ may have learning difficulties due to underlying cognitive impairment.
The NEPSY-II cognitive domain attention and executive functioning is more closely related to academic achievement than the IQ.15 Thirteen (86.6%) participants scored below average in auditory selective, sustained and divided attention, cognitive flexibility, working memory and inhibitory control. The number of omission errors was greater in the more complex activities, as seen in the performance of ten of the participants. Attention and executive functioning is reported to be the most commonly affected domain in individuals with SCD, both in those who have had a cerebral infarction and in those who have not.11 Children with attention alterations tend to perform poorly in school activities and need more time to perform tasks that require rational thought and concentration such as reading, writing and arithmetic. They often need teacher mediation during class since they are easily distracted by external stimuli. Because of their difficulty in concentrating, they fail to absorb a considerable amount of the content provided in class. Their difficulty in applying new strategies to resolve problems, in adapting to new rules and in their capacity to inhibit impulsive responses also means that these children require more time for learning. These characteristics reflect immaturity in the development of behavioral self-regulation and personal management, which also affects their personal performance, since deficits in these functions result in relationships of greater dependency with caregivers to perform routine activities.13
The Memory and Learning domain reflects the acquisition and consolidation of information.11 Results were found to be below average for verbal memory, visuospatial memory and immediate and delayed recall, with a poorer performance in visuospatial memory compared to narrative memory. Some studies have reported alterations in different memory systems.16,17 In general, less impairment was found in narrative memory with respect to immediate recognition. Academic adaptations that use this memory tool could be applied to facilitate learning at school.
Fourteen (93.3%) patients had difficulties in the language functions: comprehension of instructions, phonological processing, and word generation within specific semantic and initial letter categories. The poorest performance was in verbal comprehension. These and other aspects of language development are directly related to learning capacity and to the development of the processes of reading and writing.18 One study evaluated impairment in the semantic, syntax and phonological functions of language in individuals with SCD and suggested that they were associated with an alteration in short-term memory processes, specifically with a deficit in working memory.19 In most cases, there was impairment in both functions, which hampered the academic performance of children with SCA even further, since, in addition to the alteration in executive functioning, there were important deficits in basic language functions.
Most of the participants had some degree of impairment in visuospatial orientation and analysis and in their capacity for mental rotation. In the tests used to evaluate sensorimotor ability, writing accuracy was within the normal range in the majority of cases but there was difficulty in writing speed, motor planning and kinesthetic feedback when performance was evaluated in both hands. Some review articles have reported similar deficits.11,13 The participants had greater difficulty with activities that demanded mental planning, which is required in school work involving geometric and mechanical reasoning such as the exact sciences, possibly exerting a negative effect on their results. The speed of motor response was also found to be impaired, which may affect note taking during class. Alternatives such as the use of concrete material and audio or video recordings of classes may help these students with spatial organization and motor speed. One of the participants (Case #6), forced to use his non-dominant hand, tested within the normal range with the left hand in motor planning and kinesthetic feedback, leading to a hypothesis of adaptation based on neuroplasticity.
Seven (46.6%) of the children had repeated at least one school year and ten had a history of learning difficulties. The performance of these children was found to be poorer, particularly in the attention and executive functioning (8/10) and language domains (10/10). Alterations in these functions affect the learning ability of children with SCA, a finding that has also been reported in another study.20
The inherent complications of SCA, together with the individual's socioeconomic difficulties and their alienation from social and school life, explains the finding that the behavioral profile of individuals with SCD often includes symptoms such as anxiety/depression, alienation/depression and somatic complaints. Since SCA is a chronic disease, it represents a risk factor in psychic development and the consequent appearance of psychopathologies. Individuals with SCA suffer physical symptoms of the disease and have to cope with its numerous limitations that include restricted physical and social activities, neurological sequelae, treatment procedures and constant hospital admissions in addition to cognitive difficulties and poor academic performance. All these factors predispose children and their parents to higher levels of anxiety and depression, as shown in this and in other studies.20–22
The somatic complaints presented in seven cases may be related to chronic pain, since painful crises are common in SCA as a consequence of inflammation and infections. The symptoms of SCA explain the somatic complaints found here. Similar results were reported in another study, in which significant differences were found between the numbers of somatic complaints in children with SCA compared to unaffected children.20
There were fewer cases of problems related to the social sphere and to attention. The parents of four (26.6%) children had already identified attention problems; however, thirteen children actually had abnormalities. The behavioral evaluation of attention performed by the parents showed results that were compatible with cognitive evaluation in only three (23%) of the 13 cases, suggesting that both instruments need to be used to improve understanding of the cognitive functions to be evaluated. Disagreement with the objective finding of difficulties in the attention domain may indicate that parents/guardians fail to perceive cognitive deficits in their child. Furthermore, due to the intrinsic characteristics of the disease, parents are often relatively undemanding insofar as their child's learning is concerned or indeed may allow the child to abstain completely from school activities. This fact emphasizes the need to provide guidance on the importance of activities aimed at stimulating brain function that ultimately improve self-esteem and increase cognitive reserve.
The most common diagnoses based on Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV)23 were depressive disorder, somatic disorder and anxiety disorder. In a previous study, depression was found in 13% of children with SCD.24 The risk of the disease becoming more severe and the risk of death, as well as admissions to hospital and neurological sequelae, expose children and their families to continuous stress, increasing the risk of psychiatric disorders such as post-traumatic stress disorder, identified in three cases in this study.25 These data indicate the need to investigate emotional issues and refer patients for treatment. In addition, the family needs guidance to be able to provide support for their child, a measure that may help reduce depressive symptoms and improve the quality of life of these children.24
Based on the CBCL, none of the children fulfilled the criteria for attention deficit hyperactivity disorder (ADHD). Two studies reported similar CBCL results for children with SCD.21,26 However, one of these studies identified symptoms of ADHD in children and adolescents with SCD using a scale based on the DSM-IV.
A similar study conducted with SCA children in the 7–12 age range found significant impairment in intellectual performance, executive functioning, language, memory and visuospatial skills compared to healthy children.27 This study reported a difference of 13–14 points in the IQ score between children with SCA and controls. In addition, those with SCA were more likely to fall behind at school and to have to repeat school years as a consequence of their learning difficulties. Behavioral and emotional problems were also reported.
There was no association in this study between abnormalities in any given type of cognitive function and any specific disorder, since most of the participant's impairment was present in all the cognitive domains irrespective of the presence of a psychopathological diagnosis.
The higher mean age of the patients in this study may have played a role in the identification of greater cognitive impairment, since studies suggest an increase in impairment with increased age due to the greater predisposition to neurological events and other factors related to the disease.19,27 From a pathophysiological viewpoint, SCA evolves as vascular dementia. With time, the number of silent infarcts increases and a severe stroke often occurs, leading to further deterioration in the child's cognition. Added to this, absenteeism from school or even school dropout at an early age reduces cognitive stimulation.
When analyzed individually, Case #7 had the poorest overall performance. The patient had had a left ischemic stroke with noticeable motor (right hemiplegia) and language impairment. Neuropsychological evaluation identified reduced intellectual ability (low IQ) and deficits in all the cognitive variables evaluated.
It is interesting to note that in the two cases (#8 and #9) in which attention and executive functioning was preserved, there was no history of learning difficulties, although Case #9 had had a documented silent infarct in the past. This may reflect greater cognitive reserve and adequate maternal stimulation, since the mother in this case was attending university.
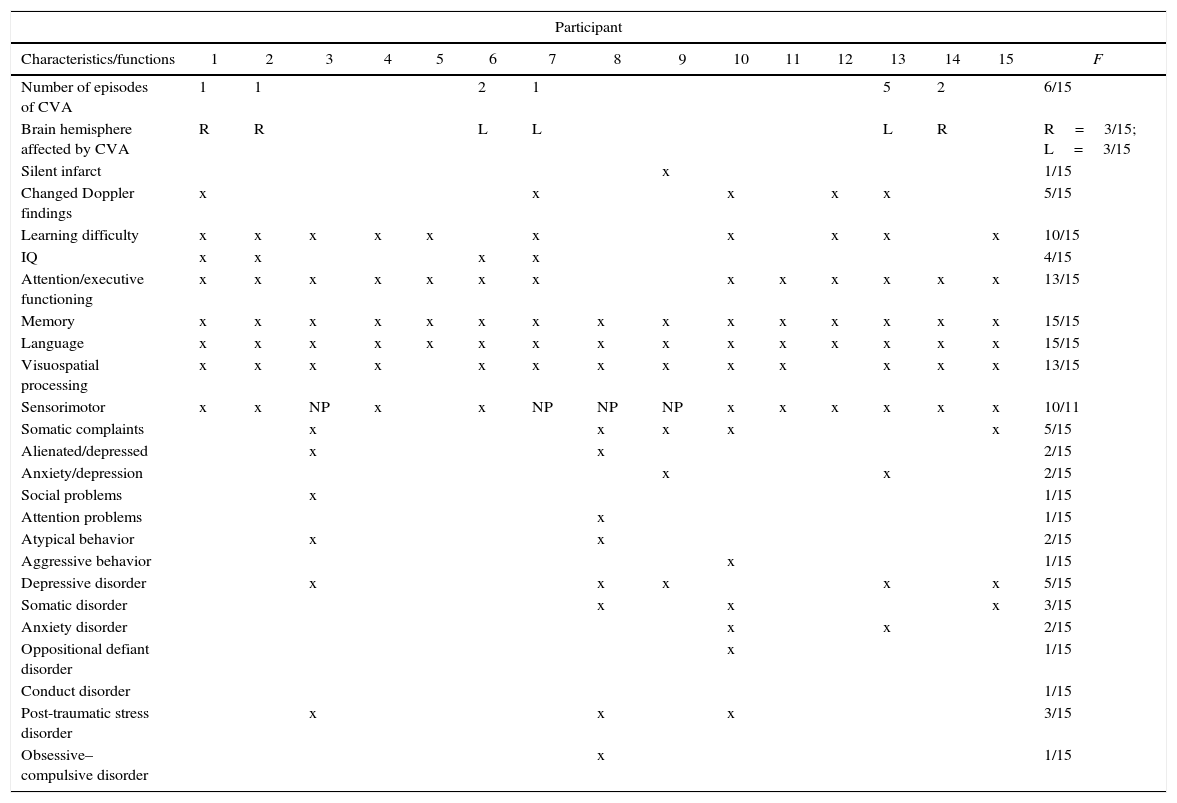
In the case-by-case data analysis summarized in Table 5, findings show that despite family follow-up and specialist medical treatment, the cognitive performance of most of the participants was poor in all the functions evaluated, emphasizing the role of SCA as a potential risk factor for neurocognitive and psychosocial development. Nine cases presented significant cognitive deficits, even those patients who had not had a stroke and those with no abnormality in maximum mean blood flow velocity (MFV). This finding suggests that in addition to the risk factors mentioned here, other risk factors inherent to the disease itself such as anemia per se and recurrent pain may contribute to poor cognitive performance. In addition, it raises the hypothesis of the existence of undiagnosed silent infarcts only found following neuropsychological evaluations.13,27,28
Summary of the results with respect to the intellectual, cognitive and behavioral performance of the individuals with sickle cell anemia and their clinical data.
| Participant | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics/functions | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | F |
| Number of episodes of CVA | 1 | 1 | 2 | 1 | 5 | 2 | 6/15 | |||||||||
| Brain hemisphere affected by CVA | R | R | L | L | L | R | R=3/15; L=3/15 | |||||||||
| Silent infarct | x | 1/15 | ||||||||||||||
| Changed Doppler findings | x | x | x | x | x | 5/15 | ||||||||||
| Learning difficulty | x | x | x | x | x | x | x | x | x | x | 10/15 | |||||
| IQ | x | x | x | x | 4/15 | |||||||||||
| Attention/executive functioning | x | x | x | x | x | x | x | x | x | x | x | x | x | 13/15 | ||
| Memory | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | 15/15 |
| Language | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | 15/15 |
| Visuospatial processing | x | x | x | x | x | x | x | x | x | x | x | x | x | 13/15 | ||
| Sensorimotor | x | x | NP | x | x | NP | NP | NP | x | x | x | x | x | x | 10/11 | |
| Somatic complaints | x | x | x | x | x | 5/15 | ||||||||||
| Alienated/depressed | x | x | 2/15 | |||||||||||||
| Anxiety/depression | x | x | 2/15 | |||||||||||||
| Social problems | x | 1/15 | ||||||||||||||
| Attention problems | x | 1/15 | ||||||||||||||
| Atypical behavior | x | x | 2/15 | |||||||||||||
| Aggressive behavior | x | 1/15 | ||||||||||||||
| Depressive disorder | x | x | x | x | x | 5/15 | ||||||||||
| Somatic disorder | x | x | x | 3/15 | ||||||||||||
| Anxiety disorder | x | x | 2/15 | |||||||||||||
| Oppositional defiant disorder | x | 1/15 | ||||||||||||||
| Conduct disorder | 1/15 | |||||||||||||||
| Post-traumatic stress disorder | x | x | x | 3/15 | ||||||||||||
| Obsessive–compulsive disorder | x | 1/15 | ||||||||||||||
NP: not performed; f: frequency; R: right; L: left; x: abnormal.
In this study, magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) were not performed due to scarcity of resources of high complexity in the Brazilian National Health System (SUS), and their high cost in the private health system. Thus, important neuroimaging data was not added to the neuropsychological evaluations of the participants in this study. This fact might explain the lower prevalence of silent ischemic strokes in this study when compared with the literature.3 Silent brain infarcts may have been underdiagnosed due to the impossibility of carrying out MRI and MRA brain scans in this sample. However, the low performance in neuropsychological assessment of patients with no apparent neurological impairment draws attention to the possibility of undiagnosed brain changes due to the inaccessibility of advanced exams in the health system and reinforces the importance of the availability of periodic imaging.
One of the limitations of the present study was the small, heterogeneous sample, which was the result of missed appointments and discontinuations due to hospitalizations, as well as other difficulties cited by the families. This made it impossible to randomize the sample, making any inferences or generalization of the results impossible.
Gaps remain in the information available on the cognition of children with SCD. Studies continue on the risk factors, predictive factors and on patients’ neuropsychological profile in an attempt to establish consensuses. New fields of research may be opened using neuropsychological investigation, including the possibility of designing studies to analyze whether the decline in cognitive and/or academic performance may be useful as a marker of outcome during treatment.
ConclusionThe findings of the present study show that individuals with SCD and a history of stroke have poorer IQ scores and abnormalities in attention and executive functioning, language, verbal and visual memory, visuospatial processing and sensorimotor ability irrespective of whether or not they had had a confirmed stroke.
This study provides considerable food for thought on the cognition of children with SCD, since findings showed significant cognitive impairment in SCA irrespective of the presence of known risk factors, emphasizing the need to investigate patients with SCD with no apparent risk factors. Few studies have targeted the emotional and psychiatric aspects of individuals with SCD and further research is needed on these issues in order to guarantee treatment for patients, reducing possible damage to their psychosocial development.
Periodic neuropsychological and behavioral evaluations of children and young adults with SCA are useful measures to reduce long-term biopsychosocial repercussions, and to stimulate participation in rehabilitation and educational programs, thus improving patients’ psychosocial development.
Conflicts of interestThe authors declare no conflicts of interest.
The authors dedicate this paper to the patients and their families.