Treatment of multiple myeloma (MM) has changed significantly in the past decade as a result of better understanding of disease biology, more effective treatments, and improved supportive care. Autologous stem cell transplantation (ASCT) is an effective treatment for myeloma and remains a critical component for its management. The goal of initial therapy remains the same, rapid disease control, and the introduction of new drugs such as thalidomide, bortezomib, and lenalidomide has enabled us to achieve this goal; combinations of these drugs have also led to unprecedented depth of response. On the other hand, the availability of these new drugs has given way to numerous double, triple, and quadruple combinations; nevertheless there is a striking paucity of randomized data to enable physicians to choose the best treatment for initial therapy. Moreover, most available randomized trials are comparisons of newer treatments against older alkylatoror anthracycline-based treatments. Thus, in the absence of randomized studies comparing different induction regimens, it is difficult to recommend one induction treatment over another.
Thalidomide is active in MM and produces little hematologic toxicity, indicating that it may be preferred for use as induction therapy. Several studies evaluating thalidomide, as a component of induction therapy, have shown that it improves response rates1-8 and progression-free survival (PFS)2,3,5,6,9 and provides similar5,6,9 or improved overall survival (OS) rates versus non-thalidomide containing treatment.3 The demonstrated efficacy, lack of myelosuppression, and overall tolerability of thalidomide provide a strong reason for its incorporation in standard induction treatment in patients with newly diagnosed MM who may be eligible for ASCT. In the last issue of the Revista Brasileira de Hematologia e Hemoterapia, the article of Crusoe et al. show the results of a retrospective study comparing pre-transplant induction therapy with conventional chemotherapy (VAD) versus thalidomide and dexamethasone (TD) or TD plus cyclophosphamide (CTD) in 152 patients with newly diagnosed MM undergoing front-line ASCT (Table 1).10 Although no differences in OS or PFS were found between the three groups, the rate of very good partial response or better response, both before and after ASCT, was higher with treatments that included thalidomide. The retrospective design of the study, the fact that only patients with at least partial response were included, and the small number of patients in each group can help explain the absence of significant differences in the follow-up. However, in the context of emerging data from ongoing trials using bortezomib and lenalidomide combinations, the improvement obtained with CTD may just not be the best that can be obtained with current therapy. Large, randomized trials are currently under way to address this and other clinically relevant questions in myeloma therapy today. Despite these limitations, untiladditional randomized data is available, the choice for initial therapy is often driven by opinion and, more importantly, by local social or economic boundaries, circumstances that have been clearly pointed out by the authors of this paper.
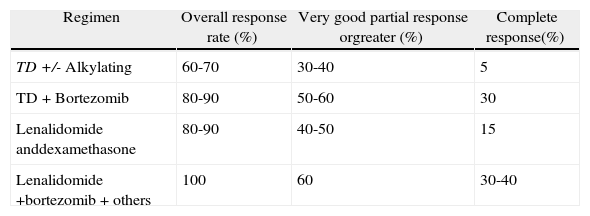
Results of different induction treatments for newly diagnosed multiple myeloma patients eligible for transplantation.
| Regimen | Overall response rate (%) | Very good partial response orgreater (%) | Complete response(%) |
| TD +/- Alkylating | 60-70 | 30-40 | 5 |
| TD+Bortezomib | 80-90 | 50-60 | 30 |
| Lenalidomide anddexamethasone | 80-90 | 40-50 | 15 |
| Lenalidomide +bortezomib+others | 100 | 60 | 30-40 |
TD: thalidomide and dexamethasone.
In summary, the results of this study clearly confirm the superiority of thalidomide-based treatments versus conventional chemotherapy for the frontline treatment of MM patients, and strongly support the use of thalidomide as part of the induction regimen in MM patients eligible for ASCT.
Conflicts of interestThe author declares no conflicts of interest.
See paper by Crusoe EQ et al, on Rev Bras Hematol Hemoter. 2014;36(1):19-24.