An important component of the advances made in neuroblastoma treatment has been the use of peripheral blood stem cells to support high-dose chemotherapy. In this study, we report our experience on a series of small children who have undergone standard and large volume leukaphersis (LVL) procedures, provide an update on a single institution's experience with cryopreservation of autologous peripheral blood stem cells (PBSCs), using 10% dimethyl sulfoxide (DMSO) and applying post-thaw DMSO depletion and analyze a number of variables that may affect viability.
MethodsA total of 36 aphereses were performed on 29 children weighing less than 25 kg between July 2016 and October 2019 at the Ibn Sina university hospital.
ResultsSeven females and twenty-two males, median bodyweight 14 kg (9 - 22). A single apheresis was sufficient to obtain at least 3 × 10⁶/kg body weight (BW) of CD34+ cells in 82.8% of the cases. The LVL was performed in 22 aphereses. A median number of 5.9 × 10⁶/kg CD34 cells were collected per apheresis. A total of 60 PBSC samples were cryopreserved and 46 samples were infused. The mean cell viability percentage decreased from 94.75 ± 1.14% before freezing to 70.84 ± 8.6% after thawing (p < 0.001). No correlation was found between post-thaw viability and storage time (r = -0.233; p = 0.234) or number of total nucleated cells (r = 0.344; p = 0.073).
ConclusionLeukapheresis is safe and feasible in small pediatric patients if the appropriate measures are used. Cryopreservation poses numerous challenges, especially a decrease in cell viability after thawing.
Neuroblastoma is an embryonal malignancy of the sympathetic nervous system; it is the third most common malignant tumor of childhood and the most common solid tumor of infancy.1 Autologous stem cell transplantation following intensive chemotherapy is a promising treatment protocol for pediatric patients with solid tumors.1 Since the development of apheresis technology, peripheral blood stem cells (PBSC) collection by apheresis has progressively replaced bone marrow as a promising and a preferred source of cells for autologous transplantation.2 Over time, numerous studies have been performed to evaluate and prove the feasibility and safety of leukapheresis in children.3-5 Successful pediatric PBSC harvesting algorithms depend on adequate vascular accesses, optimal timing of collection, adequate preparation of the child's hemodynamic and electrolyte status and an efficient apheresis procedure using appropriate measures for small children.6-7 In order to achieve the desired number of CD34+ (at least 2 × 10⁶), according to the weight of the recipient, many groups have proposed various strategies.8-9 One way to collect the target CD34+ cells and reduce the number of leukapheresis is to process a large volume leukapheresis, a procedure which implies processing the patient's total blood volume at least three times.10 Many centers over the world use this approach to increase the number of CD34+ cells harvest in poor mobilizers.10 Despite technical similarities to adult procedures, leukapheresis in pediatric patients need experienced teams, appropriate ethical safeguards and, due to the size of the patient, some procedural modifications must be considered.5,7 High-risk neuroblastoma treatment schema implies that the PBSC graft needs to be stored until use. For cryopreservation, several protocols are used by different transplant centers worldwide.11-13 This process is critical and requires the consideration of several elements, including cryoprotectant solution, storage temperature, freezing rate cell concentration and storage duration.
The aim of this study was to report our experience on a series of children weighing less than 25 Kg who had undergone standard and large volume leukapheresis (LVL) procedures using the new device Spectra Optia. The second objective was to present an update on a single institution's experience with cryopreservation of autologous peripheral blood stem cells (PBSCs) using 10% dimethyl sulfoxide (DMSO) and applying post-thaw DMSO depletion and to analyze various variables that may affect viability.
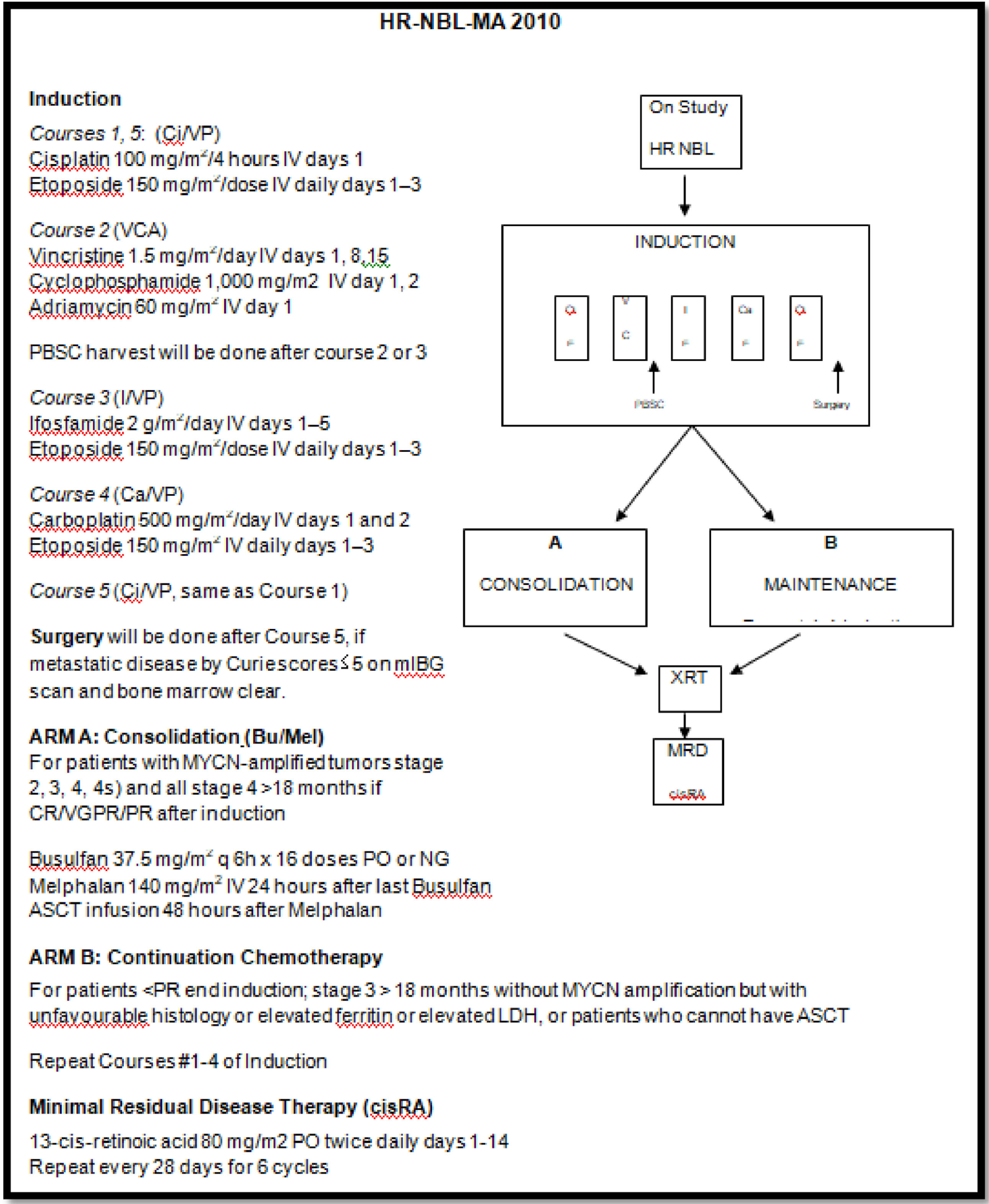
MethodsPatientsAll patients were treated according to the HR-NBL-MA 2010 protocol, which consists of neoadjuvant chemotherapy with local control of the primary tumor by surgery, consolidation by high-dose chemotherapy followed by autologous CSH and, finally, the control of residual disease by retinoic acid.14Figure 1
The protocol recommends collecting cells after the 2 to 3 cycles of chemothearpy, but this is rarely feasible due to the unavailability of collection appointments. Most often, the collection is made after 4 to 5 cycles and sometimes after the surgery.
Data were retrospectively collected on all pediatric autologous PBSC aphereses performed from July 2016 to October 2019 in our department at the Ibn Sina University Hospital. The patients included are children who weighed less than 25 kg at the time they underwent apheresis collection of PBSCs.
MobilizationBefore starting the PBSC mobilization the apheresis physician must check the eligibility of the patients. Eligibility includes a physical examination and verification of radiological (i.e., electrocardiogram and echocardiography) and laboratory results (i.e., complete peripheral blood count, calcium, potassium, magnesium, coagulation parameters, renal markers, hepatic enzyme and serology).
All collections were performed after mobilization with the granulocyte colony-stimulating factor (G-CSF), which was administered subcutaneously (5 ug/kg) twice a day for 4 days. Peripheral blood CD34+ cell counts were checked on day 4 of the G-CSF administration. Patients with pre-apheresis counts of ≥ 20 × 10⁶/L CD34+ cells were considered good mobilizers, while those with CD34+ counts < 20 × 10⁶/L were considered poor mobilizers. On day 5 of the mobilization protocol, the PBSC collection started within 2 to 3 hours following the G-CSF administration. If the required number of CD34+ cells per recipient body weight was not collected, the G-CSF administration was repeated and a second leukapheresis was performed on day 6.
Stem cell collectionLeukapheresis was performed using the continuous flow blood cell separator (Spectra Optia Terumo BCT) via a central line in the femoral, subclavian or jugular veins. Insertion sites are selected according to the patient's size and age. Placement of the central venous catheter was performed by an anesthesiologist. The Spectra Optia® apheresis system will not perform total blood volume (TBV) calculations for patients less than 25 kg in body weight; the operator must then perform manual calculation.
The extracorporeal line was primed with 200 ml of ABO and Rhesus-compatible leuko-depleted packed red blood cells before the leukapheresis to prevent dilutional anemia and to avoid a hypovolemic state. The only anticoagulant used in apheresis was citrate dextrose formulation A (ACD-A) in a ratio of 1:12. Ionized serum calcium levels were monitored before and after the procedure. To avoid hypocalcemia due to citrate intoxication, all pediatric patients received continuous calcium gluconate (1g x 10 kg body weight (BW) in 100 ml of normal saline in a slow infusion.
All patients were treated without sedation, but were diverted by activities (e.g. drawing and coloring, etc.) and the presence of a parent during the procedure. Children were monitored regularly throughout the apheresis with a cardiac monitor and a pulse oximeter. The patient's vital signs were recorded before, during and immediately after apheresis, rate flow was adjusted according to the physician's decision, depending on the patient's tolerance of the apheresis procedure.
The target yield of CD34+ cells was 5.10⁶/kg of BW, although a yield of 3.10⁶/kg of BW was considered minimally acceptable. When possible, we tried to collect more than the target number of CD34+ cells in case the patients gained weight at the time of transplantation.
SamplingThe blood count was analyzed before and after apheresis from peripheral blood using the hematology analyzer (Sysmex K21N). The quantity of CD34+ cells of both the pre-leukapheresis peripheral blood sample and the leukapheresis product were determined by flow cytometry (Cytomics FC 500, Beckman Coulter) using stem kit reagents, according to the International Society of Hematotherapy and Graft Engineering (ISHAGE) specifications.
Cryopreservation and thawing methodsThe cells were frozen within the first 24 hours after collection. All products were cryopreserved in a rate-controlled freezer for initial freezing and then stored in the nitrogen vapor phase freezers (temperature range -145 to -195 degree C°).
First, the PBSCs were transferred to a 600ml blood transfer bag and centrifuged at 870 rpm (160g) for 18 min without brake. Excess plasma was removed to obtain the appropriate cell concentration. The product was distributed in bags suitable for freezing and small aliquots were transferred to cryovials to serve as controls for the cryopreservation process.
The cryopreservation solution was prepared from hydroxyethyl starch (HES) 6% (Voluven, Fresenius Kabi, Sevres, France) and DMSO (20%) to achieve a final DMSO concentration of 10% after it was added to the apheresis products. After the addition of the cryoprotectant solution, the bags and the vials were transferred to a rate-controlled freezer (CryoMed Freezer, Thermo) as follows: The controlled rate freezing started at 4°C. This temperature was followed by a cooling rate of 2°C per minute down to -5,2°C, 45°C/min to -90°C, 25°C/min to -50°C, 10°C/min to -15°C and 2°C/min to -40°C and the procedure was completed by the final cooling rate of 10°C per minute down to a temperature of -120°C. After the cryopreservation procedure, the bags were stored in vapor nitrogen.
On the day of transplantation, the bags were thawed rapidly in a water bath at 39°C for 2 to 3 minutes. Cell viability was tested immediately after thawing. Washing procedures were performed manually with a washing solution of HES solution. After the sample was resuspended, it was centrifuged and then suspended again in normal saline before infusion.
Evaluation of cryopreservationThe number of viable nucleated cells (NCs) was evaluated by the trypan blue dye exclusion test, which was performed on both fresh and thawed samples. Nucleated cell levels were determined by a hematology analyzer, flow cytometry CD34+ cell counts were determined in aliquots collected after washing.
Statistical analysisThe IBM SPSS statistics version 20.0 for Windows was used for statistical analysis. Patient characteristics and apheresis results are described as the mean ± standard deviation or median (interquartile range) with a range. We used Spearman's correlation to test the association between the total yield of CD34+ cells and the pre-apheresis CD34+ cell count. Differences between pre-apheresis and post-apheresis platelet cell blood counts were tested with a paired samples t-test. The statistical significance of the differences between the means of viability compared was evaluated using the t-test for independent samples. The statistical significance of the differences between the medians of CD34+ cell numbers was evaluated using the Wilcoxon test for paired samples. We used the Spearman's correlation to test the association between post-thaw viability and storage time and between post-thaw viability and number of total nucleated cells.
ResultsDuring the study period, 36 peripheral stem cell apheresis procedures were performed on 29 children suffering from neuroblastoma and weighing less than 25kg. There were 22 male (75.9%) and 7 female patients (24.1%). The median age and weight were 3 years (range: 1.2 – 6.5 years) and 14kg (range: 9 – 22 kg), respectively.
In each procedure, the mean total blood volume was 1,117± 261 ml (range: 630 – 1,650 ml). Large volume leukapheresis was performed in 22 apheresis procedures and less than 3 cycles in 14 leukapheresis. A venous access was established using a dual lumen central venous catheter: in 28 procedures (77.8%) the catheter was placed in the jugular vein, in six procedures (16.7%), in the femoral vein, and in 2 (5.5%) procedures, in the subclavian vein at the time of apheresis. Technical details of PBSC collections characteristics are shown in Table 1.
Technical detail of leukapheresis and products characteristics.
| Data | value | range |
|---|---|---|
| Processed total blood volume (x)* | 3.1 ± 0.6 | 1.7 - 4.4 |
| Processed blood volume (mL)⁎⁎ | 3,414 (2,607.75; 4,323.25) | 1,356 –5,810 |
| ACD-A volume (mL)⁎⁎ | 315 (237; 390.25) | 123 - 528 |
| Procedure time (min)* | 290 ± 61 | 137 - 444 |
| CD34+ cells x 10⁶/Kg BW⁎⁎ | 5.9 (3.05; 9.55) | 0.7 - 19.7 |
ACD-A: citrate dextrose formula A; BW: body weight
A single apheresis was sufficient to obtain the desired number of CD34+ cells (at least 3 × 10⁶/kg of BW) in 24 cases (82.8%). Two aphereses were performed on consecutive days in 5 cases. Three patients needed two procedures because of insufficient numbers of CD34+cells in the first procedure, while another two children in the 24 mentioned above were reprocessed to achieve the targeted CD34+ cells (≥ 5 × 10⁶/kg of BW). Two patients were poor mobilizers and required an additional hematopoietic progenitor mobilization to obtain an adequate number of CD34+ cells.
A strong correlation, with a significant linear relationship [r = 0.714; p < 0.001], was found between the peripheral blood CD34+ cell count and the total CD34+ cell yield, considering the patient body weights obtained from the first apheresis day.
For the good mobilizers group, the pre-apheresis CD34+ cell count and product characteristics of LVL and standard apheresis on the first apheresis day are shown in Table 2. While the number of pre-apheresis CD34+ cell count was lower in good mobilizers who underwent LVL, there was no significant difference in the number of CD34+ cell counts in the stem cell product and the CD34 stem cell yield, considering patient body weights, between large volume leukapheresis and standard apheresis.
Pre-apheresis CD34+ cell count and product characteristics of LVL and standard apheresis in good mobilizers.
| LVL | Standard apheresis | p⁎⁎ | |
|---|---|---|---|
| Total number of apheresis (n)Total blood volume processing time | 153.5(3 - 4.3) | 112.5(1.7 - 2.9) | - |
| Peripheral CD34+ cell count cells/µL* | 42(22 - 188) | 64(24 - 207) | > 0.05 |
| CD34+ cells in product (cells/µL)* | 958(229 –2,484) | 979(773 – 2,173) | > 0.05 |
| CD34+ cellsx10⁶/Kg BW* | 7.07(2 - 16.5) | 6.36 (3.2 - 19.7) | > 0.05 |
LVL: large volume leukapheresis; BW: body weight
A significant decrease in platelets was observed after each leukapheresis [r = 0.937; p < 0.001], but no bleeding was observed due to the low platelet counts, whereas no significant change was observed in the hemoglobin level (p > 0.05). The median decrease in the platelets count after each leukapheresis was 40.3% (range from 26.6 - 55.28%).
No adverse events related to citrate toxicity were observed. Only four vasovagal effects were recorded related to leukapheresis, a mild and reversible decrease in blood pressure in one case at the end of the procedure and a pulse rate over 120 bpm, only at the beginning of the leukapheresis.
Laboratory characteristics of the PBSCs before and after cryopreservation are described in Table 3. A total of 60 PBSC samples were cryopreserved, with a median cellular concentration of 72.25 × 10³/µL (range 33.8 - 593; interquartile range 51.15 - 88.4).
Characteristics of PBSC sample before and after cryopreservation.
| Graft characteristics | Before cryopreservation | After cryopreservation | ||
|---|---|---|---|---|
| Value | Range | Value | Range | |
| Number of bags | 36 | 60 | ||
| Total nucleated cells (x10³/uL) | 124.21 ± 36.7* | [168 - 202] | 72.25 (51.15; 88.4)⁎⁎ | [33.8 - 593] |
| Viability (%) | 94.63 ± 1.35* | [91 - 98] | ||
| CD34+ cells number (/uL) | 842.9 (536.5;1280.78)⁎⁎ | [65 - 2484] | 499.75 (408.38;704)⁎⁎ | [65 –2,173] |
| CD34+cells (x10⁶/Kg) | 5.9 (3.05;9.55)⁎⁎ | [0.69 -19.7] | 3.03 (2.52;5.17)⁎⁎ | [0.5 - 19.7] |
| Volume of bags | 96 ± 27.48* | [25 - 180] | 121.6 ± 16.15* | [40 – 150] |
A total of 46 samples were infused. Median graft storage time was 56 days and storage time ranged between 13 and 244 days.
The DMSO was removed prior to infusion for 23 patients (44 samples), who received a mean of 4 ± 0.9 × 10⁶/Kg (range: 2 - 5.8). The viability indices show statistical differences between viability of cells in PBSC grafts assessed after collection and in post-thaw (Table 4). The mean cell viability percentage decreased from 94 ± 1.14 % before freezing to 70.84 ± 8.6% after thawing (p < 0.001). No correlation was found between the post-thaw viability and storage time (r = -0.233; p = 0.234) or number of total nucleated cells (r = 0.344; p = 0.073).
A significant difference was observed in the CD34+ cell counts between pre-freezing and post-thawing (Table 4). The mean final infusion volume was 97 ± 20ml. All products were forwarded to sterility testing and all culture results were negative.
DiscussionThe use of leukaphersis for autologous stem cell transplantation in pediatric patients continues to increase.15 Leukapheresis is efficient and safe even in small children weighing less than 25 kg, but this technique requires many preprocedural preparations.5 In this study, we retrospectively reviewed leukapheresis procedures performed in 36 sessions with 29 pediatric patients weighing 9 to 22kg.
The first challenge during stem cell collection is keeping children collaborative during the procedures,16 especially the sick children who have experienced several traumas related to their disease. Some emotional risks to the pediatric patient can be minimized by preparing the child and family for apheresis.5 In our experience, all the children were prepared for apheresis and treated without sedation and were diverted by the presence of a parent and an activity, such as coloring or watching TV.
Vascular access is of primordial importance in leukapheresis and the venous access should be adequate to maintain a constant blood flow rate needed for the procedure. For the access line in children, most authors 7,17 suggest using a double lumen dialysis catheter inserted into a femoral, jugular or subclavian vein, as we did with our patients. D. Orbach et al.18 reported that the use of a peripheral vein was sufficient to obtain an adequate blood flow rate. In our cases, we recorded no adverse events related to the use of the central venous catheter, however, several studies reported that this process can induce serious complications, such as the femoral arteriovenous fistula 18 and hemorrhage.10
Numerous centers often prefer to prime, if the extracorporeal volume exceeds 10 - 15% of the total blood volume of a child.3-4 Our practice, prior to all PBSC collections, primes the set with normal saline and anticoagulant citrate dextrose solution formula A, followed by a second prime using leukocyte-depleted compatible red blood cells and no rise back at the end of the procedure. Orbach et al. 18 reported that in many cases blood priming can be avoided in children with low weight, based on the initial hemoglobin level.
Most authors suggest the use of citrate in combination with heparin for pediatric apheresis 19 and in our series, as in the series reported by R.W. Maitta et al., 16 ACD-A alone was used. The most common adverse reaction of citrate is hypocalcemia.20-21 To avoid citrate toxicity during apheresis, most authors propose the administration of prophylactic calcium by the oral or parental route 3,17 and the pediatric calcium dose is 100 - 200 mg/Kg by IV over 5 to 10 minutes at the maximum rate of 5 ml/min.21
One of the modifiable parameters to improve stem cell collection efficiency is the volume of blood processed. To achieve the ideal collection aim, many groups recommended the use of LVL with the processing of 3 - 6 TBVs of the patients.10-22 The LVL has become more effective in increasing the number of CD34+ cells harvested, even in pediatric poor mobilizers.10 In our series, the LVL was performed in 22 apheresis procedures and less than 3 cycles were performed in 14 leukaphereses. Our results show that both a standard apheresis procedure and LVL are feasible and that a single apheresis was sufficient to obtain the desired number of CD34+ cells (at least 3 × 10⁶/Kg of BW) in 82.8% of the cases. Another factor positively affecting the CD34+ collection efficiency is the circulating peripheral blood CD34+ cell concentration one day prior to, or on the day of, the stem cell harvest.23 In our series, 86% of the patients were good mobilizers and blood CD34+cell counts strongly correlate with the total CD34+ cell yield, considering the patient body weights. In accordance with the literature, the pre-apheresis CD34+ cell count is still the best indication to initiate the PBSC collection.24,25
Like other authors,10,26 we observed decreased platelet counts after each leukapheresis, however, no complication related to thrombocytopenia has been reported in our cases. In our protocol, to avoid any bleeding risk for the patient, we started apheresis when the platelet count was above 90 × 10⁹/L.
The high-risk neuroblastoma treatment schema implies that the PBSC graft needs to be stored until use. For cryopreservation, several protocols are used by different transplant centers worldwide. This process is critical and requires the consideration of several points.
Pre-freeze storage after the collection of cells may affect the cryopreservation outcome. Guttridge et al. reported that delays in the cryopreservation time significantly influence the viability of CD34+ cells from umbilical cord blood (UCB) after cryopreservation.26 Cryopreservation within 48 hours or less is recommended to ensure the optimum cell viability.28
Much of the research in this area focuses on the Identification of critical points in the process.
Volume reduction is the first step before cryopreservation of the hematopoietic stem cells (HSCs). This procedure must ensure the high recovery of viable cells. The total nucleated cell recovery after volume reduction should be > 75%.13
It has been identified that a high cell concentration at freezing is a possible cause of cell loss. Based on the literature review, we consider the maximum NC concentration in the cryostored transplant should not exceed 4 × 10⁸/ml.27
The DMSO at 10% in the final concentration is the most used component in a freezing solution including other additives, such as human albumin or saline serum. The cryoprotective action of DMSO is to prevent cell damage during the freezing and thawing processes.13 Previous washing for the purpose of DMSO depletion is routinely performed at our center to minimize patient adverse reactions.
Another factor that can cause significant loss of cell viability is thawing. According to our data, thawing the cryopreserved cells resulted in a significant decrease in the CD34+ cell counts and viabilities. This decrease in CD34+ cells and cell viability were similar to the rates reported previously.28
In our series, as in several the other studies,28-29 no correlation was found between post-thaw viability and storage time.
This study was limited by the sample size analyzed and data on the hematopoietic recovery in the post-transplant period were not available. However, we want to share the experience at our center.
ConclusionDespite technical similarities to adult procedures, leukapheresis in small children still worries some operators over the world that have little experience in managing pediatric patients. The present manuscript can serve as a comparative experience and encourage other apheresis teams to start leukapheresis with children.
Finally, we can conclude that leukapheresis remains safe for, and well tolerated by, small children without sedation, even using LVL if the appropriate measures are used. The most important factors are peripheral blood CD34+ cell concentration one day prior to, or on the day of, the stem cell harvest, adequate preparation of the child regarding the electrolyte status and emotional stress, a good venous access and the management of the extracorporeal volume of the disposable. The cryopreservation process is critical. In this article, we reviewed several variables that may affect the quality of the stem cell graft.