Mantle cell lymphoma (MCL) is a rare non-Hodgkin's lymphoma with an average age of 66 years. The most common involvements of non-lymphoid tissues are the digestive tract and liver.1 Central nervous system (CNS) involvement is quite rare among patients with MCL.2,3 We present a patient who has been diagnosed as dementia initially because of cognitive and behavioral symptoms. However, generalized seizures were observed after three months and we found a CNS involvement of MCL.
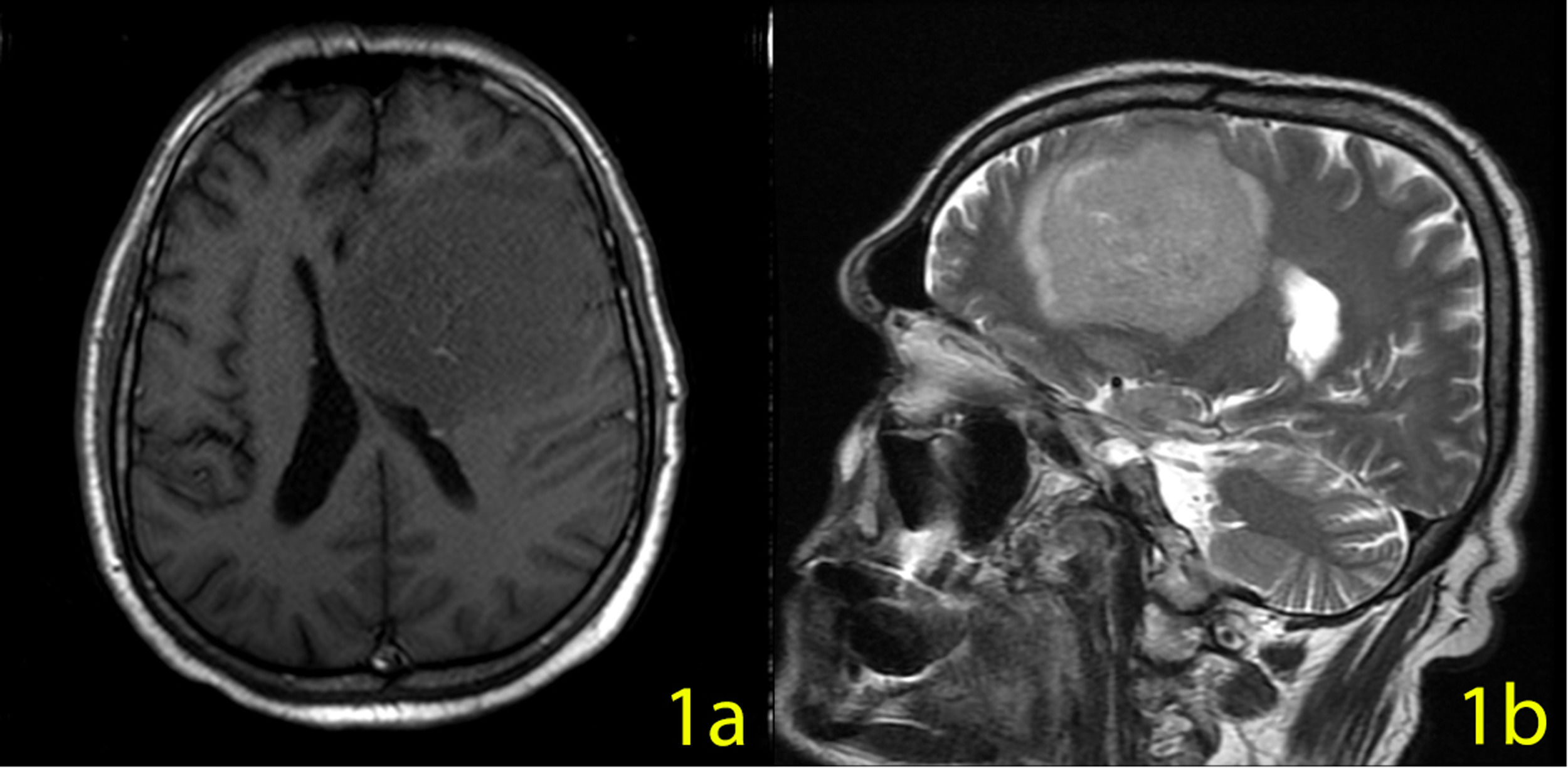
Case: A 60-year-old man admitted to the emergency department with generalized convulsion. In the cranial MRI, a 70×65×62 mm-sized lesion was detected in the left frontal lobe with a more prominent contrast enhancement at the center of the lesion (a significant finding for lymphoma) (Figure 1a,b). In his clinical history, cognitive, depressive, and behavioral symptoms were prevalent. His primary symptoms were irritability, loss of interest, apraxia, and amnesia. He was diagnosed with dementia and depressive disorder. A typical antipsychotic and an antidepressant drug were started in the psychiatry department. He continued this treatment for three months until his first seizure.
The patient was diagnosed with advanced MCL 8 years ago, and the R-CHOP protocol was applied. Early-stage relapse was detected after 2 years of remission. As a rescue treatment, 4 cycles of R-CHOP were applied. After complete response, the patient went through an autologous blood stem cell transplantation. He then followed in remission for 6 years.
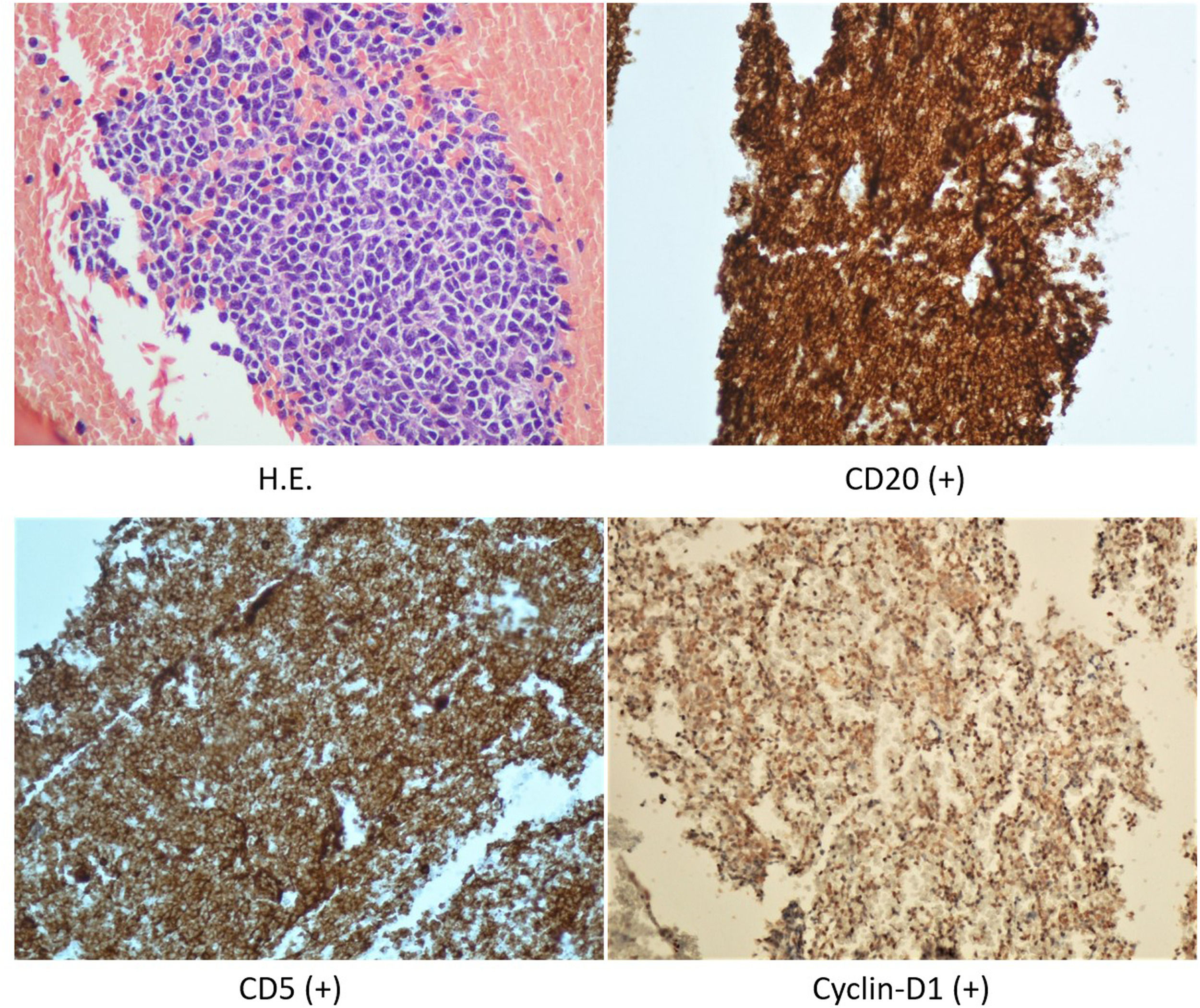
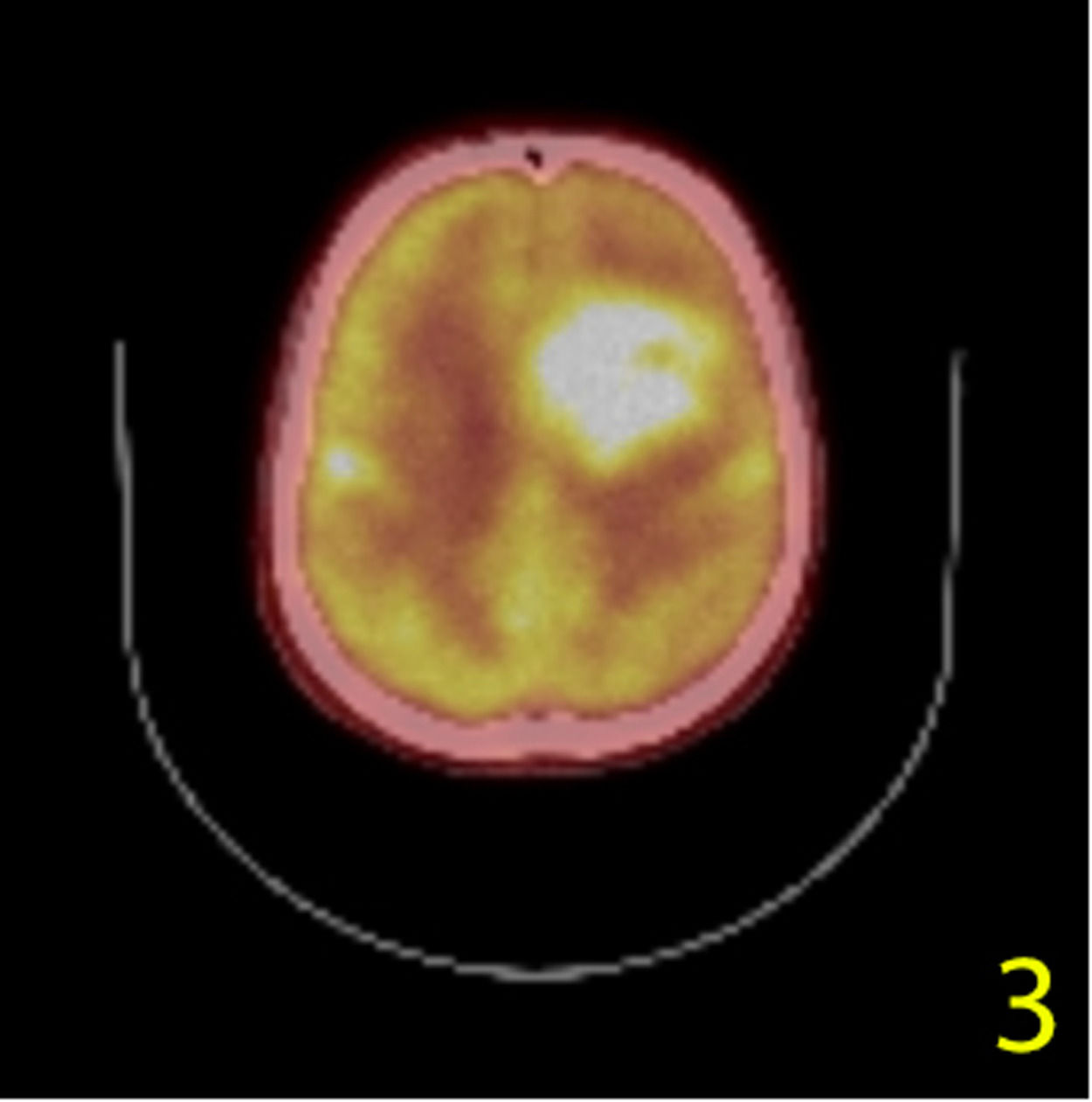
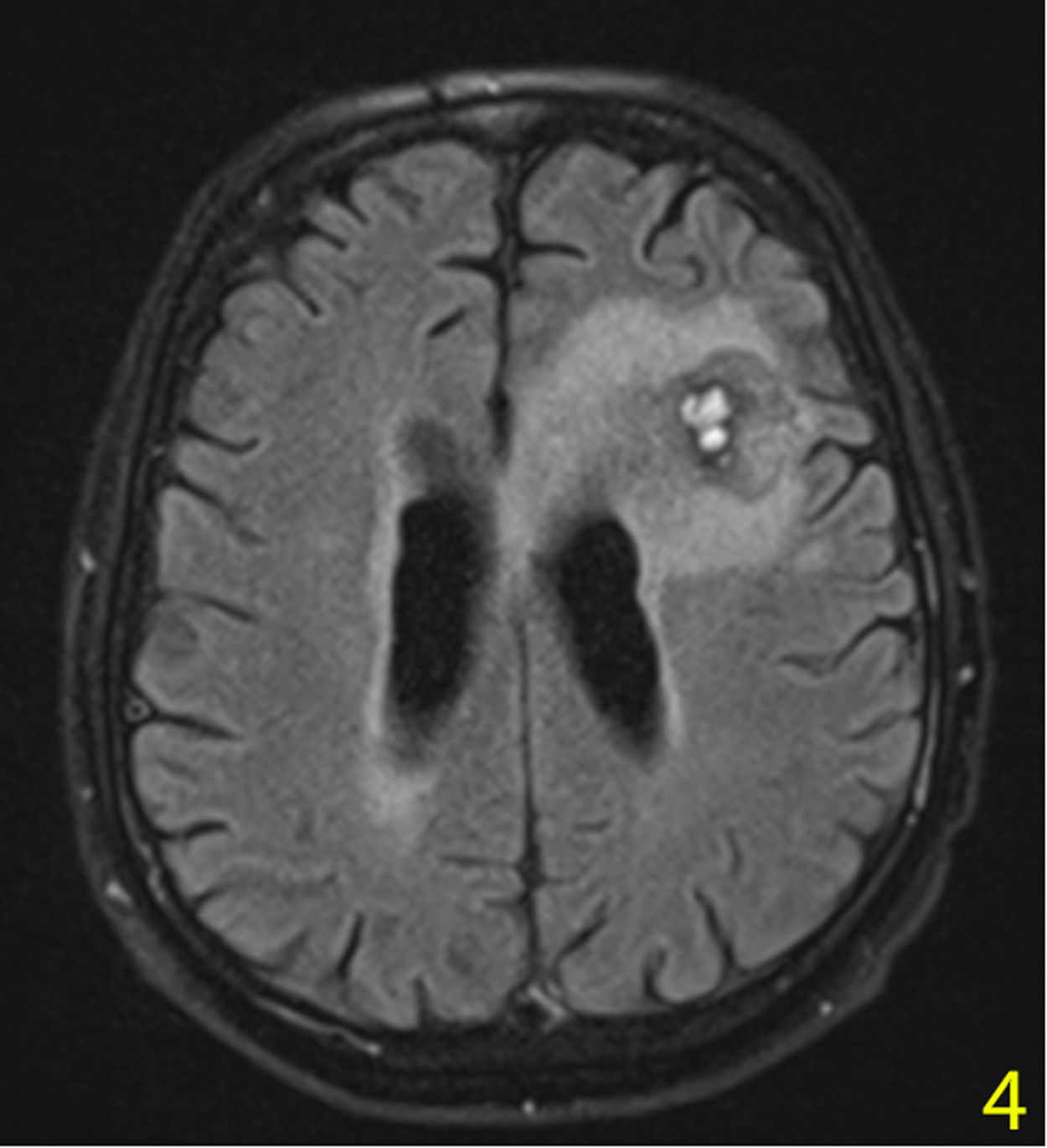
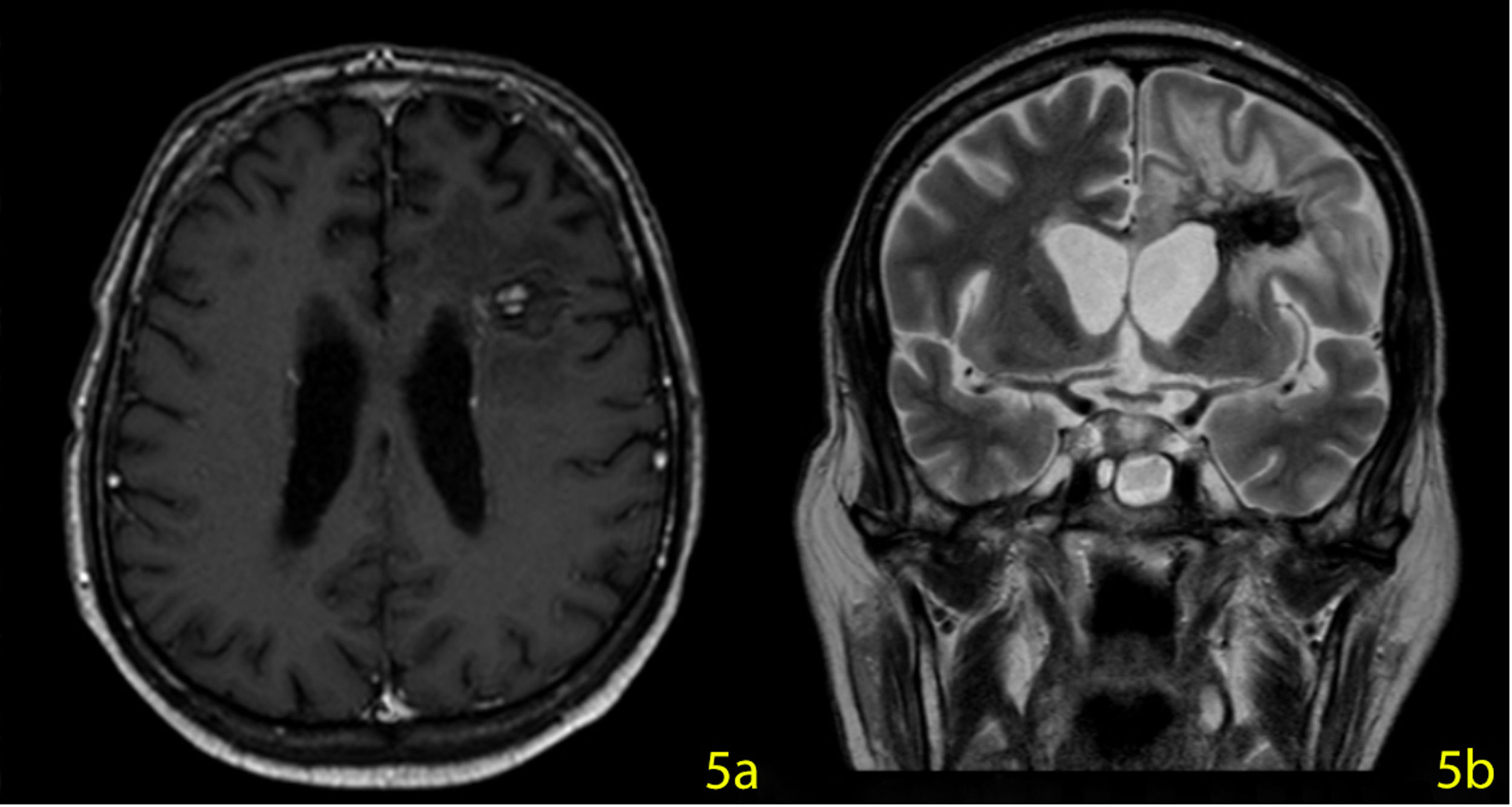
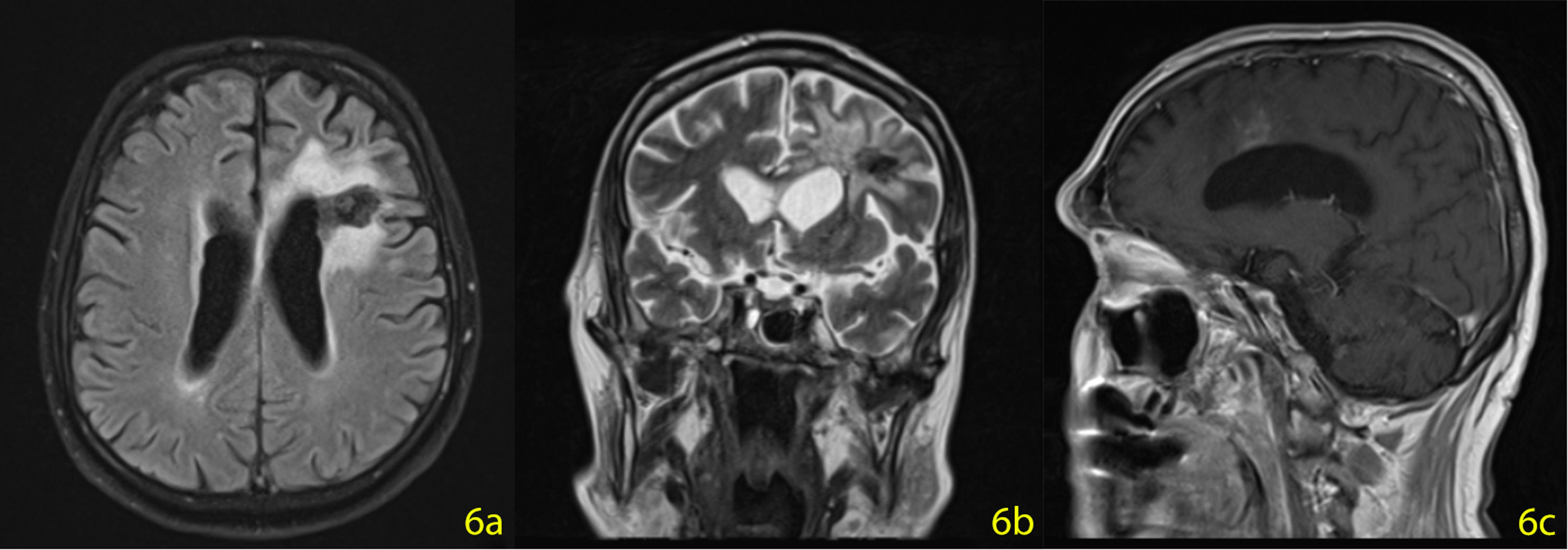
A stereotactic biopsy was made for histological diagnosis. Immunohistochemistry analysis demonstrated that CD20, CD5, and Cyclin-D1 were positive (Figure 2). The patient was re-evaluated with the diagnosis of MCL from the brain parenchymal tissue. PET-CT showed no other involvement rather than the brain parenchyma (Figure 3). The patient, who was diagnosed with isolated CNS MCL, was given MATRix (methotrexate, cytarabine, thiotepa, rituximab) combination chemotherapy, especially known for its efficacy in MCL and CNS lymphoma. On the 8th day of the regimen, the mass was reduced in the cranial MRI (40×34×20 mm) (Figure 4). On the 25th day of the regimen, his alertness increased, he started to communicate in the form of single words. His nutrition and movements improved. The MRI repeated on the 30th and 45th day, the mass was regressed even further (36×25×17mm and 23×22.5×15mm respectively) (Figure 5a,b, Figure 6a,b,c).
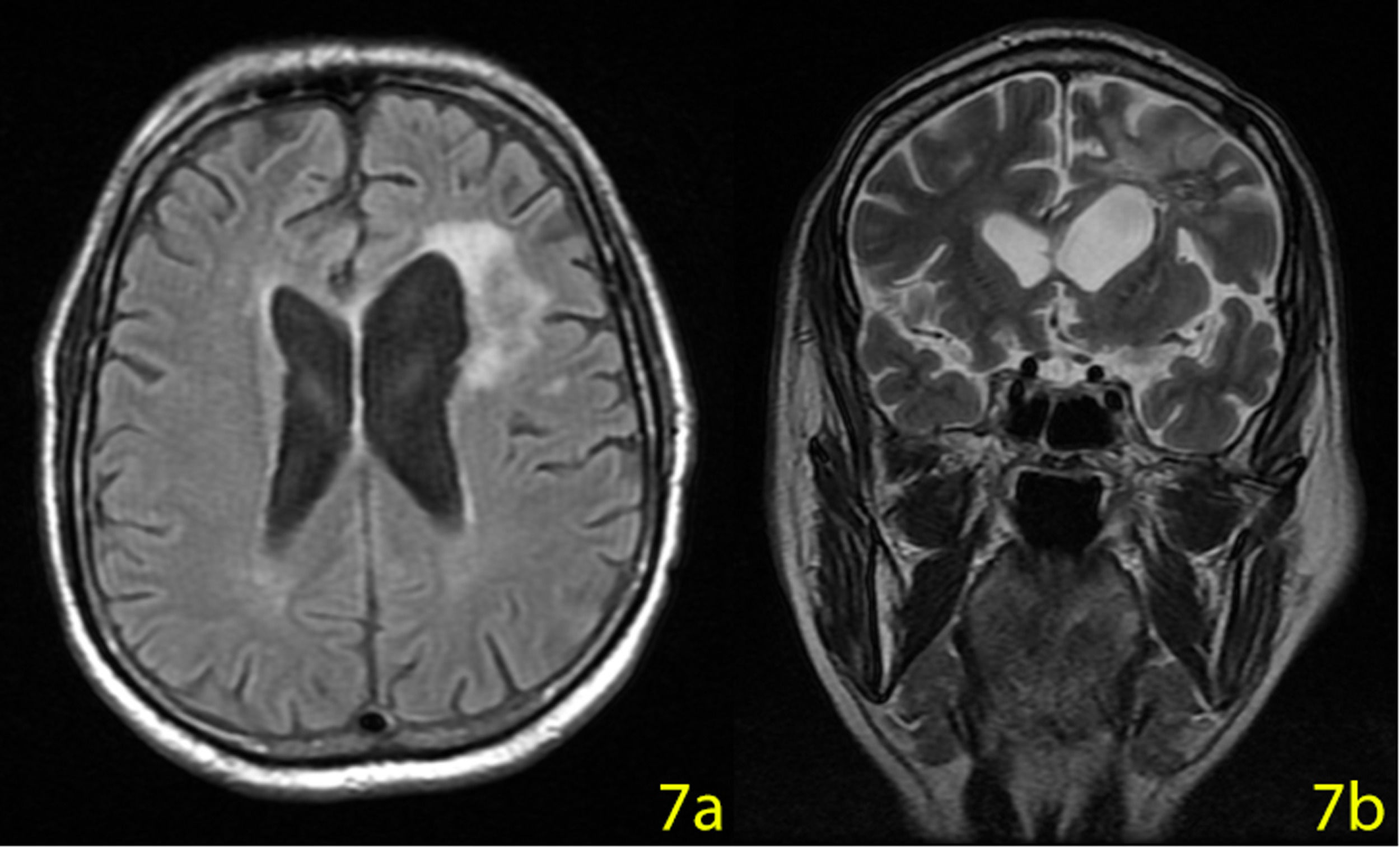
On the 55th day of the treatment, the patient was discharged from the hospital and Ibrutinib 560 mg/day started. An MRI performed on the 210th day of the treatment. The mass disappeared leaving a 20×15 mm malasic area with peripheral gliosis (Figure 7a,b). The patient is now in complete remission and continuing Ibrutinib treatment until further relapses.
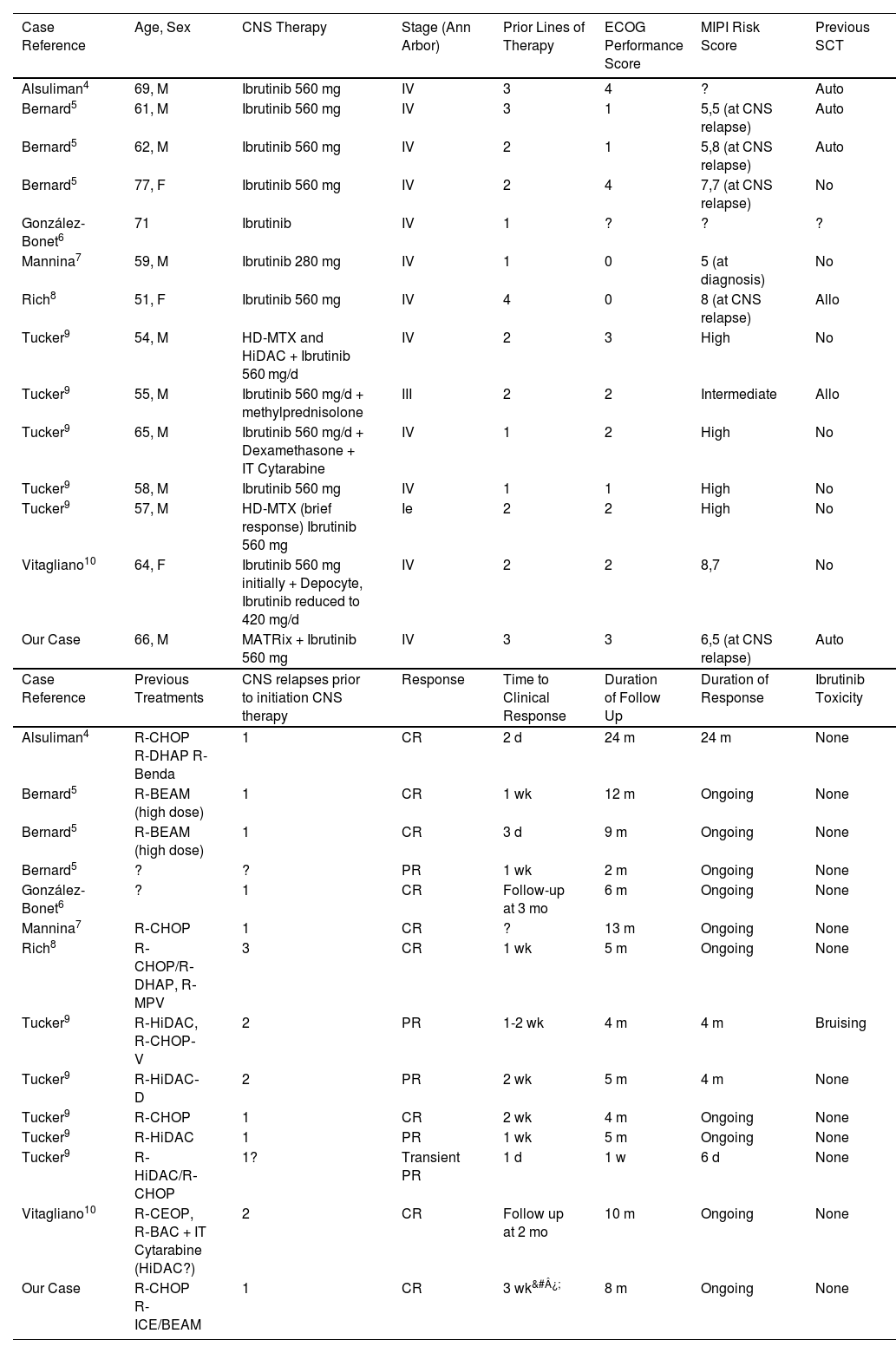
We have found 14 more cases of CNS relapses who have used Ibrutinib in the literature (Table 1).
CNS relapses of MCL who have used Ibrutinib in the literature
| Case Reference | Age, Sex | CNS Therapy | Stage (Ann Arbor) | Prior Lines of Therapy | ECOG Performance Score | MIPI Risk Score | Previous SCT |
|---|---|---|---|---|---|---|---|
| Alsuliman4 | 69, M | Ibrutinib 560 mg | IV | 3 | 4 | ? | Auto |
| Bernard5 | 61, M | Ibrutinib 560 mg | IV | 3 | 1 | 5,5 (at CNS relapse) | Auto |
| Bernard5 | 62, M | Ibrutinib 560 mg | IV | 2 | 1 | 5,8 (at CNS relapse) | Auto |
| Bernard5 | 77, F | Ibrutinib 560 mg | IV | 2 | 4 | 7,7 (at CNS relapse) | No |
| González-Bonet6 | 71 | Ibrutinib | IV | 1 | ? | ? | ? |
| Mannina7 | 59, M | Ibrutinib 280 mg | IV | 1 | 0 | 5 (at diagnosis) | No |
| Rich8 | 51, F | Ibrutinib 560 mg | IV | 4 | 0 | 8 (at CNS relapse) | Allo |
| Tucker9 | 54, M | HD-MTX and HiDAC + Ibrutinib 560 mg/d | IV | 2 | 3 | High | No |
| Tucker9 | 55, M | Ibrutinib 560 mg/d + methylprednisolone | III | 2 | 2 | Intermediate | Allo |
| Tucker9 | 65, M | Ibrutinib 560 mg/d + Dexamethasone + IT Cytarabine | IV | 1 | 2 | High | No |
| Tucker9 | 58, M | Ibrutinib 560 mg | IV | 1 | 1 | High | No |
| Tucker9 | 57, M | HD-MTX (brief response) Ibrutinib 560 mg | Ie | 2 | 2 | High | No |
| Vitagliano10 | 64, F | Ibrutinib 560 mg initially + Depocyte, Ibrutinib reduced to 420 mg/d | IV | 2 | 2 | 8,7 | No |
| Our Case | 66, M | MATRix + Ibrutinib 560 mg | IV | 3 | 3 | 6,5 (at CNS relapse) | Auto |
| Case Reference | Previous Treatments | CNS relapses prior to initiation CNS therapy | Response | Time to Clinical Response | Duration of Follow Up | Duration of Response | Ibrutinib Toxicity |
| Alsuliman4 | R-CHOP R-DHAP R-Benda | 1 | CR | 2 d | 24 m | 24 m | None |
| Bernard5 | R‐BEAM (high dose) | 1 | CR | 1 wk | 12 m | Ongoing | None |
| Bernard5 | R‐BEAM (high dose) | 1 | CR | 3 d | 9 m | Ongoing | None |
| Bernard5 | ? | ? | PR | 1 wk | 2 m | Ongoing | None |
| González-Bonet6 | ? | 1 | CR | Follow-up at 3 mo | 6 m | Ongoing | None |
| Mannina7 | R‐CHOP | 1 | CR | ? | 13 m | Ongoing | None |
| Rich8 | R‐CHOP/R‐DHAP, R‐MPV | 3 | CR | 1 wk | 5 m | Ongoing | None |
| Tucker9 | R‐HiDAC, R‐CHOP‐V | 2 | PR | 1-2 wk | 4 m | 4 m | Bruising |
| Tucker9 | R‐HiDAC‐D | 2 | PR | 2 wk | 5 m | 4 m | None |
| Tucker9 | R‐CHOP | 1 | CR | 2 wk | 4 m | Ongoing | None |
| Tucker9 | R‐HiDAC | 1 | PR | 1 wk | 5 m | Ongoing | None |
| Tucker9 | R‐HiDAC/R‐CHOP | 1? | Transient PR | 1 d | 1 w | 6 d | None |
| Vitagliano10 | R‐CEOP, R‐BAC + IT Cytarabine (HiDAC?) | 2 | CR | Follow up at 2 mo | 10 m | Ongoing | None |
| Our Case | R-CHOP R-ICE/BEAM | 1 | CR | 3 wk&#¿; | 8 m | Ongoing | None |
Cognitive and affective symptoms were manifested initially in our case. We found an isolated CNS involvement which is a rare clinical form of MCL. CNS involvement at MCL is 0.9% at the time of diagnosis, and the overall involvement rate is 4%.2,3 In cranial tumors, especially frontal, temporal and/or parietal lobe involvement, personality changes, speech and memory disorders may occur. Cranial tumors should be taken into consideration in the differential diagnosis of cognitive, affective, and behavioral disorders.
The authors would like to thank Dr. Damla Çalışkan for preparing the pictures.