Early Death (ED) remains challenging in newly diagnosed acute promyelocytic leukemia (APL), especially in developing countries. The clinical and laboratory profile at diagnosis were evaluated and causes and risk factors were investigated in adult APL patients.
MethodA retrospective real-life analysis of 141 medical records was performed of patients diagnosed with APL between 2007 and 2018, whether they were treated with the IC-APL 2006 protocol or not. Risk factors were assessed by univariate and multivariate analysis.
Main resultsOverall, 112 patients were included in the study. ED occurred in 22.3% of cases, surpassing clinical trial reports, with non-protocol-eligible patients presenting notably higher rates (60%), potentially due to their clinical status. Hemorrhage (60%) and infection (33.3%) were the leading causes of ED. Univariate analysis associated ED to the ECOG score; white blood cell (WBC) count; body mass index; levels of hemoglobin, albumin, uric acid, and creatinine, aPTT and INR and FLT3 mutations. Multivariate analysis identified ECOG score ≥2 and elevated WBC count as independent risk factors.
ConclusionED remains a substantial challenge in APL, especially in real-world settings with hemorrhage and infection being the leading causes. ECOG status and WBC count emerged as independent risk factors, while age and platelet count lacked a 30-day prognostic correlation. Evaluating prognostic enhancement tools in controlled trials and real-life settings is pivotal to improving APL outcomes.
Acute promyelocytic leukemia (APL), or acute myeloid leukemia with t(15;17), is distinguished from other leukemia subtypes through its unique clinical and morphological features; it is the subtype with the most favorable outcomes in modern times.1 APL is a pioneering model of acquired genetic diseases treated with a specific drug, all-trans-retinoic acid (ATRA), which induces terminal differentiation followed by apoptosis of leukemic cells. The combination of ATRA with arsenic trioxide (ATO) currently guarantees overall survival rates greater than 90%.2–5 However, there is still a substantial occurrence of early death (ED), defined as death within the first 30 days after diagnosis, especially in developing countries, with this remaining the main obstacle to improving prognosis.6–9
Affecting 60% to 90% of patients and often serving as the primary cause of ED, disorders of hemostasis are prevalent in APL with central nervous system (CNS) hemorrhage being a significant cause of unfavorable outcomes. 10,11 Several prognostic factors have been identified in APL, like elevated white blood cell (WBC) count and advanced age, but discrepancies persist between populations.7,10,12–18 Other variables have also been associated with ED, however more studies are necessary to better understand the prognostic factors in APL induction therapy.
In this article, the clinical and laboratory profiles at diagnosis were evaluated and causes and risk factors for ED were investigated in a cohort of adult APL patients over a 12-year period. Patients were treated in one of the eight Brazilian centers participating in the International Consortium on Acute Promyelocytic Leukemia (IC-APL).
Material and methodsStudy design and patient selectionA retrospective chart review was performed of all the 141 patients with APL registered at the Hemope Foundation between January 2007 and December 2018. Hemope is a state reference blood center for diagnosing and treating hematologic diseases in Recife, PE, Brazil. A total of 112 patients were included in the study for analysis whether they were eligible for the IC-APL 2006 protocol (n = 97) or not (n = 15). The main reasons for exclusion from the protocol were treatment in another hospital (n = 10), no molecular diagnosis (n = 9), younger than 18 years old (n = 3), loss to follow-up (n = 2), and mislaid charts (n = 2).
Treatment protocolsThe IC-APL 2006 protocol adapted from PETHEMA LPA 2005 was used, however treatment with idarubicin was replaced by the more cost-effective daunorubicin. 19 Patients were selected for the IC-APL treatment protocol based on eligibility criteria: age 18–75 years old at diagnosis; Eastern Cooperative Oncology Group (ECOG) performance status ≤3; morphological diagnosis of APL or promyelocytic leukemia-retinoic acid receptor-α (PML-RARα) rearrangement. The PML-RARα rearrangement was confirmed by reverse transcription polymerase chain reaction (RT-PCR). Patients who met these criteria but had one of the following characteristics received alternative treatment protocol adaptations accordingly: associated psychiatric or neoplastic disease, seropositivity for the human immunodeficiency virus, serum creatinine ≥3.5 mg/dL or clinical contraindication for anthracycline.
Treatment was initiated promptly upon diagnostic suspicion. The induction therapy consisted of ATRA and daunorubicin, and the consolidation therapy consisted of three courses of treatment as described by Rego et al., 19 transfusion support aimed at maintaining a platelet count >30–50×109/L and a fibrinogen level >15 g/L. Differentiation syndrome prophylaxis was made with dexamethasone for 15 days in patients who presented WBC counts >5.0 × 109/L within the first two weeks.
Ethical considerationThis study was approved by the Hemope Ethics Committee and was carried out in accordance with the Declaration of Helsinki. For this study, the signing of informed consent forms was waived, as patients had already given their consent for the main IC-APL project.
Statistical analysesData from medical records were analyzed using SPSS 13.0. Measures of central tendency and dispersion presented numerical variables. The Student's t-test was used to compare standard distribution variables and Mann-Whitney for non-normal data. Fisher's exact test and Pearson chi-square test were used for crossing of categorical variables. The multivariate analysis used logistical regression (ENTRE method) and included variables with p-values ≤0.20 in the bivariate analysis. All the tests were applied with 95% confidence intervals.
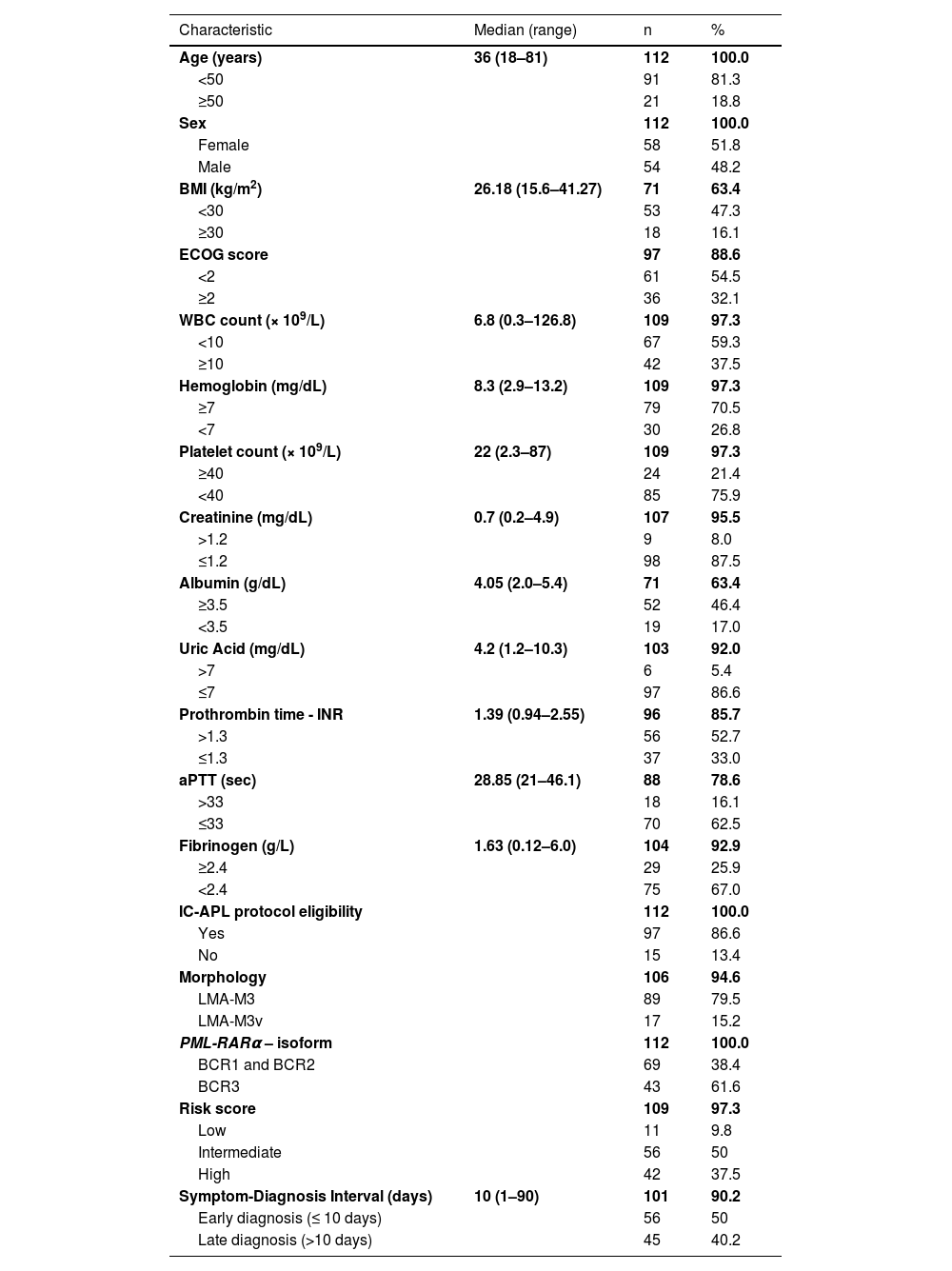
Results and discussionPatient characteristicsA total of 112 patients were analyzed (Table 1); they were predominantly young, with a median age of 36 (range: 18–81) years, as reported by earlier studies. 11,13,19–21 Interestingly, more recent studies have reported older ages at diagnosis, with median ages varying from 44 to 54 years. 7–9,17,18,22 This divergence might be attributed to the prevalence of database studies in more recent literature, unlike the previous clinical trial studies that tend to exclude factors that could heighten patient risk, such as older age. Consistent with existing literature, no significant sex differences were found.5,7–9,17,22 Most records analyzed belonged to patients eligible for the IC-APL protocol. Among the 15 patients not eligible for the protocol, most exhibited clinical features that rendered them unqualified with the main reason for protocol ineligibility being ECOG performance status of 4.
Clinical and laboratory characteristics of the study population.
BMI: Body Mass Index; ECOG score: Eastern Cooperative Oncology Group performance status; WBC: White Blood Cell; INR: International Normalized Ratio; aPTT: Activated partial thromboplastin time.
All patients had anemia and low platelet counts at diagnosis; WBC count varied from leukopenia to severe leukocytosis. This classic APL presentation is well-established, though a higher WBC count is considered a poor prognostic marker.2,7,11,13–17 Patients were categorized as low, intermediate, and high risk as per the risk model of Sanz et al.23 Symptom-diagnosis time spanned 1–90 days (interquartile range: 7–15 days).
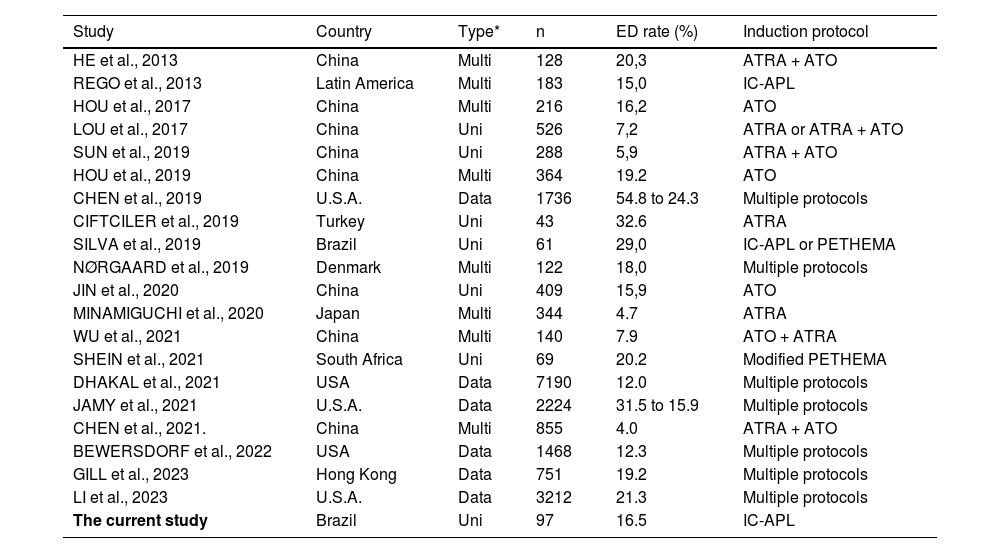
Early death and its causesThe overall ED rate was 22.3%. Among patients eligible for the IC-APL protocol, the ED rate was 16.5%, whereas non-eligible patients exhibited a much higher rate of 60%. Studies on single-drug treatment protocols10–15,19,21,24,25 and database studies7–9,22,26 reported 12–32.6% ED rates (Table 2). Notably, centers using the ATRA and ATO combo in multiagent therapy achieve 4.0–7.9% ED rates.2,4,5,7,13 The association between both drugs has also been reported as a factor for better overall and disease-free survival.2,4,5,7,13
The early death rates studies reported.
| Study | Country | Type* | n | ED rate (%) | Induction protocol |
|---|---|---|---|---|---|
| HE et al., 2013 | China | Multi | 128 | 20,3 | ATRA + ATO |
| REGO et al., 2013 | Latin America | Multi | 183 | 15,0 | IC-APL |
| HOU et al., 2017 | China | Multi | 216 | 16,2 | ATO |
| LOU et al., 2017 | China | Uni | 526 | 7,2 | ATRA or ATRA + ATO |
| SUN et al., 2019 | China | Uni | 288 | 5,9 | ATRA + ATO |
| HOU et al., 2019 | China | Multi | 364 | 19.2 | ATO |
| CHEN et al., 2019 | U.S.A. | Data | 1736 | 54.8 to 24.3 | Multiple protocols |
| CIFTCILER et al., 2019 | Turkey | Uni | 43 | 32.6 | ATRA |
| SILVA et al., 2019 | Brazil | Uni | 61 | 29,0 | IC-APL or PETHEMA |
| NØRGAARD et al., 2019 | Denmark | Multi | 122 | 18,0 | Multiple protocols |
| JIN et al., 2020 | China | Uni | 409 | 15,9 | ATO |
| MINAMIGUCHI et al., 2020 | Japan | Multi | 344 | 4.7 | ATRA |
| WU et al., 2021 | China | Multi | 140 | 7.9 | ATO + ATRA |
| SHEIN et al., 2021 | South Africa | Uni | 69 | 20.2 | Modified PETHEMA |
| DHAKAL et al., 2021 | USA | Data | 7190 | 12.0 | Multiple protocols |
| JAMY et al., 2021 | U.S.A. | Data | 2224 | 31.5 to 15.9 | Multiple protocols |
| CHEN et al., 2021. | China | Multi | 855 | 4.0 | ATRA + ATO |
| BEWERSDORF et al., 2022 | USA | Data | 1468 | 12.3 | Multiple protocols |
| GILL et al., 2023 | Hong Kong | Data | 751 | 19.2 | Multiple protocols |
| LI et al., 2023 | U.S.A. | Data | 3212 | 21.3 | Multiple protocols |
| The current study | Brazil | Uni | 97 | 16.5 | IC-APL |
Beyond multi-drug availability, socioeconomic factors also influence ED rates, as evidenced by worse outcomes in developing nations21,25 and disadvantaged areas in developed countries. 8,9,17,22,26 Real-life studies also tend to present higher ED rates, probably due to the restrictive conditions of clinical trials and the selective nature of patient recruitment. 8,9,17,18,22 In this study, all patients ineligible for the IC-APL protocol were included, and their worse clinical status might explain the higher ED rate.
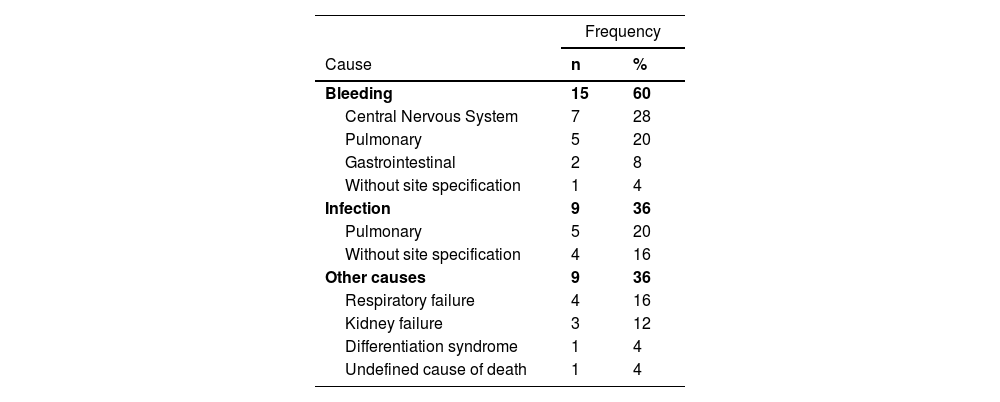
ED was caused mainly by hemorrhage and infection (Table 3). CNS was the primary bleeding site with two patients presenting concomitant intracranial hypertension. The lungs were the second major site for bleeding and the first for infection. ED was also related to respiratory and kidney failures; only one patient died from differentiation syndrome. The high incidence of hemorrhagic in EDs is expected because of the classical clinical presentation of the disease with coagulopathy. Lethal hemorrhage within the first 30 days is often reported as the main cause of death, with CNS bleeding accounting for 50–92.3% of ED rates.2,5,7,10–14,21,25
Concomitant causes of the 25 early deaths in the study.
The percentages of hemorrhagic and infectious causes of death varies based on many factors. Jin et al. found that the proportion of infectious ED was higher in older patients (>50 years old) compared to younger patients (30.3% vs. 3.1%), while the hemorrhagic ED rate was significantly lower (48.5% vs. 87.5%; p-value = 0.0006), possibly because of the worse clinical status of the elderly group, especially regarding the immune system. 11,19 Time of death also matters, as most hemorrhagic deaths occur within the first seven days of diagnosis and infectious EDs happen between the 7th and 30th day with a higher incidence of sepsis-related death described in the consolidation stage of treatment. 11,21,25 This might be related to a low tolerance to treatment protocols, as hemorrhagic deaths are attributed to the disease itself, whereas infectious deaths are also associated with immune impairment from chemotherapy side effects. This study took place at a blood center, which is known to offer hematology and blood transfusion services in the same building; it even has a specialized intensive care unit (ICU). This might have reduced the incidence of ED due to coagulopathy and differentiation syndrome.
Socioeconomic factors might play a role in infectious ED rates. Silva et al. found that all patients treated at an academic center in Brazil experienced febrile neutropenia during induction, with a high incidence of positive blood cultures and evidence of fungal infections. 21 Developed countries and China usually show lower infectious death rates (0–17.4%) than developing nations (33–52%) such as Brazil and South Africa.5,7,12,21,25 This might be due to population characteristics, epidemiological distribution of resistant microorganisms and the less common use of multi-drug treatment, such as ATO, which is not widely available in these countries.
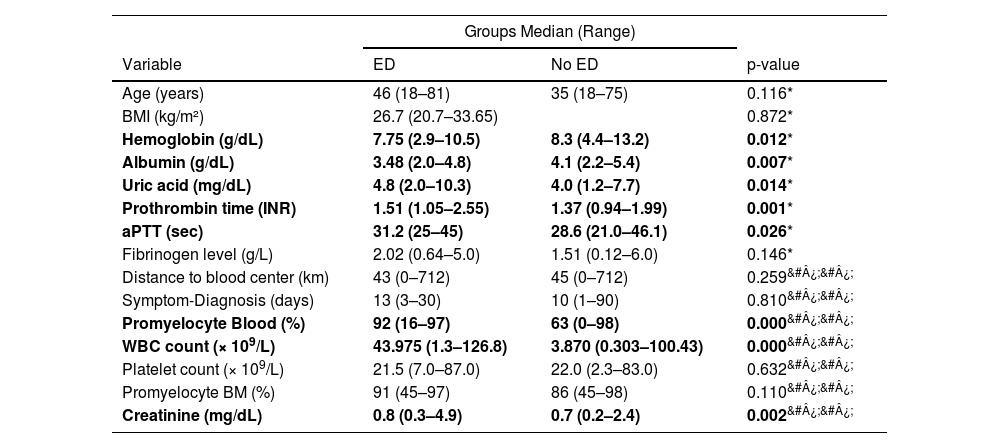
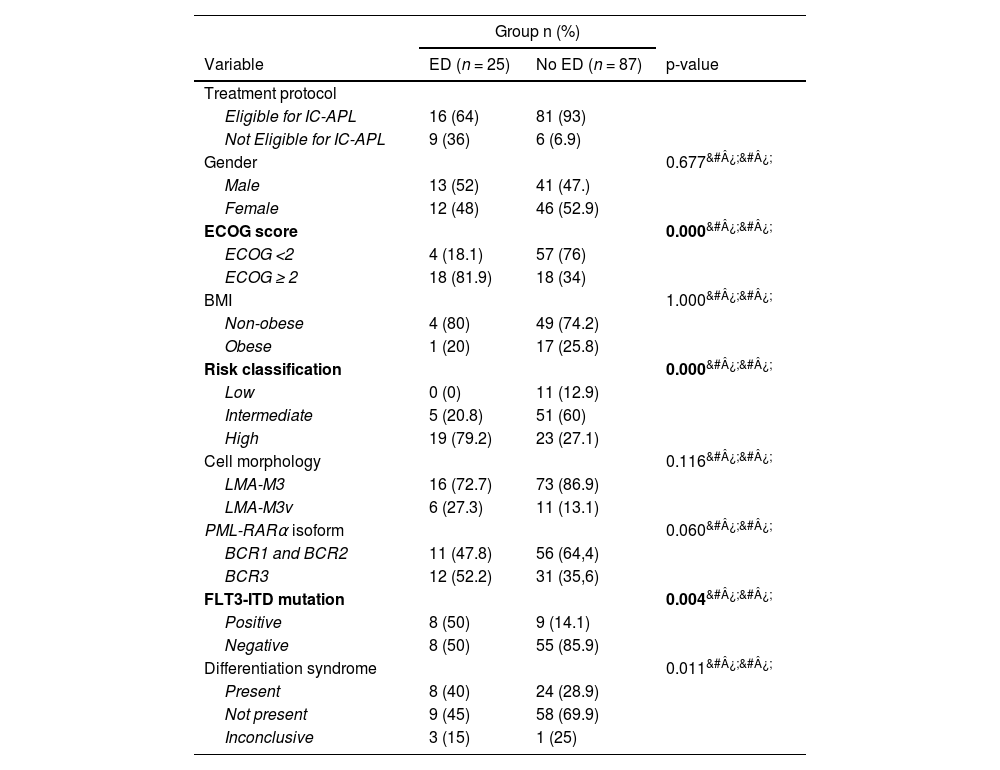
Risk factorsUnivariate analysisThe group of patients who presented ED had significantly higher BMI, uric acid, activated partial thromboplastin time, International Normalized Ratio, creatinine, and WBC count, besides lower hemoglobin and albumin levels in the univariate analysis (Table 4). The patients in the ED group also presented higher ECOG scores and more frequent FLT3 mutations (Table 5). These findings align with the literature, as these are risk factors reported in multiple studies.2,5,16,17,22,24,26,7,8,10–15
Continuous variables of patients with and without early death.
| Groups Median (Range) | |||
|---|---|---|---|
| Variable | ED | No ED | p-value |
| Age (years) | 46 (18–81) | 35 (18–75) | 0.116* |
| BMI (kg/m²) | 26.7 (20.7–33.65) | 0.872* | |
| Hemoglobin (g/dL) | 7.75 (2.9–10.5) | 8.3 (4.4–13.2) | 0.012* |
| Albumin (g/dL) | 3.48 (2.0–4.8) | 4.1 (2.2–5.4) | 0.007* |
| Uric acid (mg/dL) | 4.8 (2.0–10.3) | 4.0 (1.2–7.7) | 0.014* |
| Prothrombin time (INR) | 1.51 (1.05–2.55) | 1.37 (0.94–1.99) | 0.001* |
| aPTT (sec) | 31.2 (25–45) | 28.6 (21.0–46.1) | 0.026* |
| Fibrinogen level (g/L) | 2.02 (0.64–5.0) | 1.51 (0.12–6.0) | 0.146* |
| Distance to blood center (km) | 43 (0–712) | 45 (0–712) | 0.259&#¿;&#¿; |
| Symptom-Diagnosis (days) | 13 (3–30) | 10 (1–90) | 0.810&#¿;&#¿; |
| Promyelocyte Blood (%) | 92 (16–97) | 63 (0–98) | 0.000&#¿;&#¿; |
| WBC count (× 109/L) | 43.975 (1.3–126.8) | 3.870 (0.303–100.43) | 0.000&#¿;&#¿; |
| Platelet count (× 109/L) | 21.5 (7.0–87.0) | 22.0 (2.3–83.0) | 0.632&#¿;&#¿; |
| Promyelocyte BM (%) | 91 (45–97) | 86 (45–98) | 0.110&#¿;&#¿; |
| Creatinine (mg/dL) | 0.8 (0.3–4.9) | 0.7 (0.2–2.4) | 0.002&#¿;&#¿; |
ED: Early death; BMI: Body mass index; aPTT: Activated partial thromboplastin time; INR: International Normalized Ratio; WBC: White blood count; BM: Bone Marrow.
Categorical variables of patients with and without early death.
| Group n (%) | |||
|---|---|---|---|
| Variable | ED (n = 25) | No ED (n = 87) | p-value |
| Treatment protocol | |||
| Eligible for IC-APL | 16 (64) | 81 (93) | |
| Not Eligible for IC-APL | 9 (36) | 6 (6.9) | |
| Gender | 0.677&#¿;&#¿; | ||
| Male | 13 (52) | 41 (47.) | |
| Female | 12 (48) | 46 (52.9) | |
| ECOG score | 0.000&#¿;&#¿; | ||
| ECOG <2 | 4 (18.1) | 57 (76) | |
| ECOG ≥ 2 | 18 (81.9) | 18 (34) | |
| BMI | 1.000&#¿;&#¿; | ||
| Non-obese | 4 (80) | 49 (74.2) | |
| Obese | 1 (20) | 17 (25.8) | |
| Risk classification | 0.000&#¿;&#¿; | ||
| Low | 0 (0) | 11 (12.9) | |
| Intermediate | 5 (20.8) | 51 (60) | |
| High | 19 (79.2) | 23 (27.1) | |
| Cell morphology | 0.116&#¿;&#¿; | ||
| LMA-M3 | 16 (72.7) | 73 (86.9) | |
| LMA-M3v | 6 (27.3) | 11 (13.1) | |
| PML-RARα isoform | 0.060&#¿;&#¿; | ||
| BCR1 and BCR2 | 11 (47.8) | 56 (64,4) | |
| BCR3 | 12 (52.2) | 31 (35,6) | |
| FLT3-ITD mutation | 0.004&#¿;&#¿; | ||
| Positive | 8 (50) | 9 (14.1) | |
| Negative | 8 (50) | 55 (85.9) | |
| Differentiation syndrome | 0.011&#¿;&#¿; | ||
| Present | 8 (40) | 24 (28.9) | |
| Not present | 9 (45) | 58 (69.9) | |
| Inconclusive | 3 (15) | 1 (25) | |
ED: Early death; IC-APL: International Consortium on Acute Promyelocytic Leukemia ECOG: Eastern Cooperative Oncology Group; PML-RARα: promyelocytic leukemia-retinoic acid receptor-α; BMI: Body mass index.
Age was not significantly different between the groups in the current cohort, despite its widely described role as a risk factor in other populations. Jin et al. found that older age was an independent risk factor11 while Sun et al. reported age as the sole independent risk factor in a Chinese academic center population. 2 Similar findings were described in the literature. 12,13,26 Nevertheless, Hou et al. reported that age did not pose an increased risk for thrombohemorrhagic ED in patients treated with single ATO therapy. 14 Similarly, Ciftciler et al. found that age was not a predictor of ED. 15 This collective evidence, in line with the results of this study, suggests the need for further investigation of the role that age plays in the prognosis of APL patients during induction therapy, including considering the number of individuals in published studies.
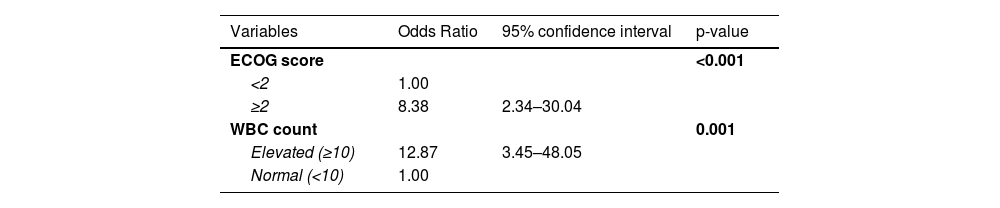
Multivariate analysisThe variables included in the multivariate analysis encompassed eligibility for the treatment protocol, ECOG score, age, presence of differentiation syndrome, cell morphology, PML-RARα isoform, WBC and platelet count, fibrinogen and creatinine serum level.
We found that ECOG performance status and WBC count were independent predictors for ED in the analyzed population (Table 6). Patients with higher ECOG scores have overall worse clinical characteristics and have consistently reported associations with ED.10,15,24
Many studies have described the WBC count as an independent risk factor for ED and two risk scores have been conceived based on this premise.7,10,12–18 Lou et al. proposed an adaptation of the Sanz Risk Score for relapse, in which WBC, platelet level and age classified patients as low, intermediate, high and ultra-high risk for ED. In parallel, Österroos et al. also suggested using the same parameters, but dividing patients into low, high and very high-risk groups, with promising results in its validation cohort. It is noteworthy that although this study did find WBC as an independent risk factor for ED, the current cohort did not show a correlation between ED and either age or platelet levels, raising concerns about the validity of these risk scores within the current population.
ConclusionThe overall ED was 22.3%, and, as such, it remains a challenge to manage recently diagnosed APL, especially in real-world settings, as the group of patients who were not eligible for the IC-APL protocol presented an appalling 60% ED rate. The leading cause of death within 30 days of disease was hemorrhage, followed by a high infection rate. This trend is reported in other developing countries, suggesting the role of epidemiological and socioeconomic factors in such fatalities. ECOG performance status and WBC count were independent risk factors for ED, whereas age and platelet count did not present a correlation with the 30-day prognosis. More extensive studies are imperative to validate possible risk scores in our population, as ED is still the main obstacle for better outcomes in APL, especially in developing countries. Such tools, allied with multiagent therapy, might help improve the prognosis of APL patients.