Febrile neutropenia (FN) is a serious complication of cancer chemotherapy. The present study aimed to identify risk factors for documented infection in pediatric patients with FN and cancer.
MethodsThis prospective cohort study included patients under 18 years from 2016 to 2018. Infection was defined according to the Centers for Disease Control and Prevention criteria.
ResultsA total of 172 febrile neutropenic episodes were evaluated. From univariate analysis, the risk factors were: female gender; monocyte count < 100 cell/mm³, platelets < 50,000, C-reactive protein (CRP) > 90 mg/dl and hemoglobin < 7mg/dl at the onset of an episode; two or more episodes of FN, and; fever onset; positive blood culture at the fever onset. Independent risk factors according to the multivariate analysis were: CRP at the onset of a febrile episode > 90mg/dl, fever onset and first blood culture with a positive result. The lowest probability of infection was related to first episode and to platelets > 50,000 at the onset of fever.
ConclusionA CRP > 90 at the onset of a febrile episode, platelets < 50,000, second episode or more, first fever episode during hospitalization and positive first blood culture were found to be associated with a higher risk of infection and they could be useful for the establishment of risk scores for infection in neutropenic children.
Febrile neutropenia (FN) is an oncological emergency and a serious complication of cancer chemotherapy.1,2 Patients with suppressed immune systems might present fever as the only sign of an underlying infection. According to previous studies in the last 20 years, rates of bacteremia in this population range from 6% to 60%. The predominant infectious foci are the lower respiratory tract, urinary tract and gastrointestinal tract.3-8 Infections confirmed by clinical or microbiological tests account for less than half of all the cases of FN and most children present no symptoms during treatment.9
Among pediatric patients with FN, various risk factors have been associated with infectious complications, such as the type of cancer, intensity of the chemotherapy, duration and degree of neutropenia, platelet count and C-reactive protein value.4,10-12 Nevertheless, depending on the demographic location, some of these factors might suffer variations.
Considering that the demographic location should be considered in the risk stratification of infectious complications among pediatric patients with FN,2,13 the present study aimed to identify the risk factors for documented infection in children and adolescents with FN and cancer at a reference pediatric unit. A secondary objective was to describe the clinical, laboratory and microbiological profile of pediatric patients hospitalized and undergoing cancer treatment during episodes of FN.
Material and methodsDesign and locationThis was a prospective study conducted at the Pediatric Inpatient Unit of the Hospital das Clínicas of the Federal University of Minas Gerais (UFMG), Belo Horizonte, Minas Gerais, Brazil. The Ethical Committee approved the study, which was developed from June 2016 to June 2018.
Inclusion criteriaWe enrolled children and adolescents under 18 years old diagnosed with cancer and FN. FN was defined as an axillary temperature > 38°C in a single measurement or > 37.8°C for at least one hour or two measurements within 24 hours and neutrophil count < 500 cells/mm³ or between 500 - 1000 cells/mm³, with a predicted decline. Patients were monitored from the time of the FN diagnosis until the termination of the antibiotics use. Eligible patients were identified by a daily blood count test and the presence of fever in medical records. Patients with hematopoietic cell transplants were excluded because they had been admitted in a different section of the hospital. All patients who agreed to participate in the study previously signed an Informed Consent Form (ICF).
Data collection and follow-up careDemographic, clinical and laboratory data of the patients at the onset of FN were collected: age, sex, type of neoplasia, absolute neutrophil count, absolute monocyte count, platelet count, CRP, hemoglobin, first episode of FN, place where first fever occurred (at home or in the hospital), first positive blood culture test and presence of central catheter. The first blood culture was collected for screening of infection at the beginning of the episode of febrile neutropenia.
The outcome variable (dependent) was defined as the documented infection.
All patients were diagnosed and treated according to the established protocol at the Pediatric Inpatient Unit, which includes, anamnesis and comprehensive physical exams, propaedeutic investigation with blood count, CRP, blood culture, routine urine analysis and urine culture. Empiric antimicrobial therapy was conducted with fourth generation cephalosporin.
Infection criteriaWe used the standard infection criteria of the National Healthcare Safety Network (NHSN) of the Centers for Disease Control and Prevention (CDC)14 for bacterial infections and the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC / MSG)15 for invasive fungal infections. Thus, infections were divided into 8 groups: fever with no determined infectious focus; bloodstream infection (laboratory-confirmed bloodstream infection (LCBI) and mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI)); Clostridioides difficile infection; neutropenic enterocolitis; invasive fungal infection (proved, probable a possible invasive fungal infection); skin and soft tissue infection; lower respiratory tract infections, and; genitourinary tract infections. The signs and symptoms of upper respiratory infection were considered as other infections.
Microorganism identification method and sensitivity testThe microbiological isolation was performed by an automated method (VITEK2) and the resistance profile was determined by the disk diffusion test (Kirby Bauer). The sensitivity profile of microorganisms was established by the Hospital Infection Control Commission (CCIH), based on the Clinical and Laboratory Standards Institute (CLSI).16
Statistical analysisThe mean, median, quartiles and standard deviation were obtained through descriptive analysis. The variables of interest were described by a 95% confidence interval (95%CI). The association between two categorical variables was performed using the Pearson's chi-square test and Fisher's exact test. For significant differences (p < 0.05) between groups, the degree of association between two categorical variables was calculated by the Odds Ratio. The Student's t-test compared the means between children with and without infection. Homogeneity of variance was verified by the Levene test. The Logistic Regression model identified variables that jointly influenced the occurrence of documented infections and the Hosmer and Lemeshow fit evaluated the fit of the model to the survey data. The Cox & Snell and Nagelkerke pseudo-R² measurements verified if the variables were sufficient to explain the outcome. The categorical variable with more than two categories (degree of neutropenia > 500, between 500 and 100 and < 100) was dichotomized and transformed into a Dummy variable. All results were considered significant for a significance probability of less than 5% (p < 0.05).
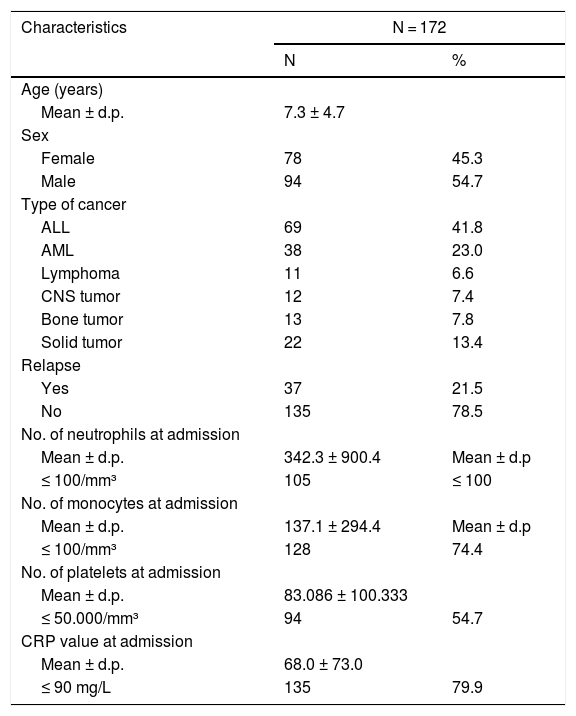
ResultsPopulation characteristicsThe 172 episodes of FN in 77 patients were evaluated, with an average of 1.64 episodes per patient. The mean age of the children at the onset of an episode was 7.3 years and 54.7% of the FN episodes were in male patients. The distribution of the underlying diseases was as follows: 68.6% of the population was diagnosed with leukemia and lymphoma and 31.4% had a solid tumor. Patients with tumor relapse corresponded to 21.5% of the study population.
The mean absolute neutrophil count at the beginning of the episode was 342.3 cells/mm³ and 61% of the cases had severe neutropenia (< 100 cells/mm³). The mean absolute monocyte count at the beginning of the episode was 137.1 cells/mm³ and 74.4% presented a monocyte count less than < 100 cells/mm³. The mean CRP was 68 mg/L and 79.9% of the cases presented CRP > 90 mg/L in the first measurement.
Of all the cases, 19% were the first episode of FN induced by chemotherapy and 53.2% experienced the fever onset at home. The average duration of the fever, hospitalization and antibiotic therapy were 13.1, 41.7 and 19.7 days, respectively. A total of 31 (18%) patients had septic shock and 21 (12.2%) were transferred to the Intensive Care Unit. The mortality rate was 4.7% (8 patients). The clinical and demographic data are summarized in Table 1.
Demographic and clinical features of pediatric cancer patients with febrile neutropenia in a reference pediatric unit from June 2016 to June 2018.
ALL: acute lymphoblastic leukemia, AML: acute myeloblastic leukemia, CNS: central nervous system, CRP: C-reactive protein.
The microorganisms were isolated from 82 blood cultures; gram-positive bacteria were isolated from 41.2% of positive blood cultures, gram-negative were isolated in 49.6% and fungi, in 7.2% of the cultures. The most prevalent bacteria were Staphylococcus epidermidis (13.4% of the positive blood cultures), followed by Pseudomonas aeruginosa (12.1%) and Klebsiella pneumoniae (10.9%). The candida species was the only species of fungi isolated from the blood. The most common pathogen from the urine cultures was Klebsiella pneumoniae (4 urine samples). The pathogens isolated from the skin cultures were Acinetobacter baumannii (1 case) and Fusarium sp. (3 cases). Resistant pathogens corresponded to 30.9% of the analyzed sterile-site cultures.
Among the episodes of FN, 56 (32.5%) presented resistant bacteria colonization and/or infection. In 14 cases (25%), resistant enterobacteria were identified, 14 (25%) were extended-spectrum beta-lactamase-producing bacteria (ESBL), 11 (19.6%) were multiresistant Acinetobacter sp., 9 (16%) were Enterococcus sp. Vancomycin-resistant (VRE), 7 were (12.5%) multidrug-resistant Pseudomonas sp. and 1 case (1.8%) was Stenotrophomonas maltophilia.
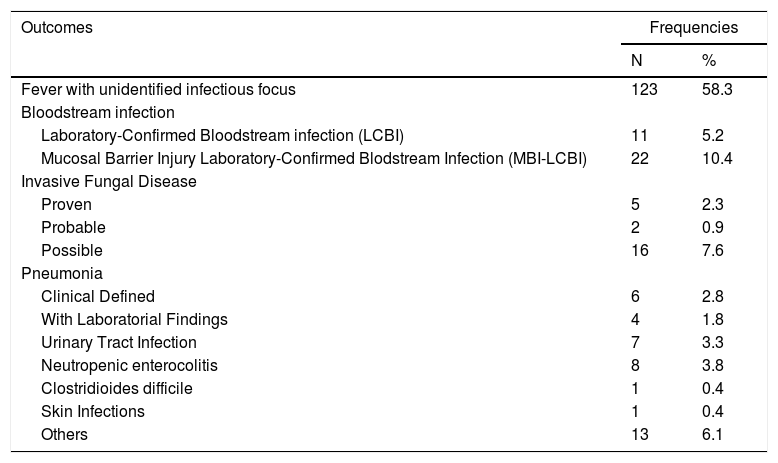
Fever with unidentified infectious focus was the most frequent clinical diagnosis, corresponding to 58.3% of the febrile neutropenia episodes. A bloodstream infection occurred in 15.6% of the episodes; 22 (10.4%) of the bloodstream infections were related to a mucosal barrier breakdown. There were episodes with multiple confirmed infections.
The remaining 5 possible types of infections accounted for less than 10% of the cases and we considered the signs and symptoms of upper respiratory infections as other infections, accounting for 6.1% of the febrile neutropenia episodes (Table 2).
Infections in pediatric cancer patients with febrile neutropenia in a reference pediatric unit from June 2016 to June 2018.
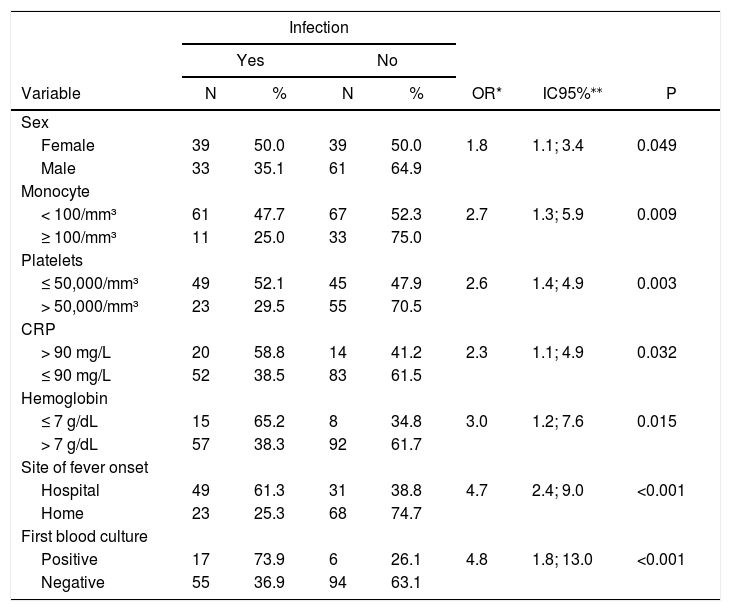
From univariate analysis, the risk factors presenting a statistically significant association (p < 0.05) with an infection outcome were: female, absolute monocyte count < 100 cell/mm³ at the onset of the episode, platelet count < 50,000 at the onset of the episode, CRP > 90 mg/dl at the onset of the episode, hemoglobin < 7 mg/dl at the onset of the episode, two or more episodes of febrile neutropenia, a fever onset during hospitalization and a positive first blood culture (Table 3).
Predictive factors for infection in pediatric cancer patients with febrile neutropenia in a reference pediatric unit from June 2016 to June 2018: univariate analysis.
| Infection | |||||||
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| Variable | N | % | N | % | OR* | IC95%⁎⁎ | P |
| Sex | |||||||
| Female | 39 | 50.0 | 39 | 50.0 | 1.8 | 1.1; 3.4 | 0.049 |
| Male | 33 | 35.1 | 61 | 64.9 | |||
| Monocyte | |||||||
| < 100/mm³ | 61 | 47.7 | 67 | 52.3 | 2.7 | 1.3; 5.9 | 0.009 |
| ≥ 100/mm³ | 11 | 25.0 | 33 | 75.0 | |||
| Platelets | |||||||
| ≤ 50,000/mm³ | 49 | 52.1 | 45 | 47.9 | 2.6 | 1.4; 4.9 | 0.003 |
| > 50,000/mm³ | 23 | 29.5 | 55 | 70.5 | |||
| CRP | |||||||
| > 90 mg/L | 20 | 58.8 | 14 | 41.2 | 2.3 | 1.1; 4.9 | 0.032 |
| ≤ 90 mg/L | 52 | 38.5 | 83 | 61.5 | |||
| Hemoglobin | |||||||
| ≤ 7 g/dL | 15 | 65.2 | 8 | 34.8 | 3.0 | 1.2; 7.6 | 0.015 |
| > 7 g/dL | 57 | 38.3 | 92 | 61.7 | |||
| Site of fever onset | |||||||
| Hospital | 49 | 61.3 | 31 | 38.8 | 4.7 | 2.4; 9.0 | <0.001 |
| Home | 23 | 25.3 | 68 | 74.7 | |||
| First blood culture | |||||||
| Positive | 17 | 73.9 | 6 | 26.1 | 4.8 | 1.8; 13.0 | <0.001 |
| Negative | 55 | 36.9 | 94 | 63.1 | |||
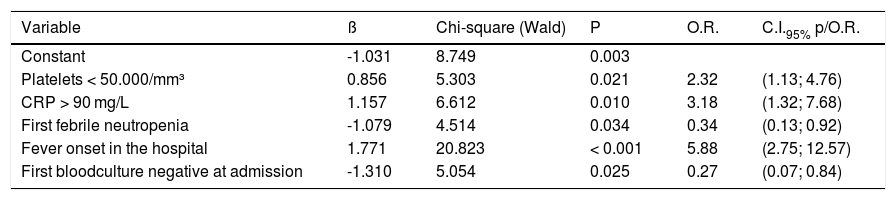
The variables that remained in the multivariate model associated with infection were: a platelet count at the onset of episode ≤ 50,000 (2.32 odds ratio (OR), 95%CI 1.11; 4.76, p = 0.021), CRP > 90 mg/dL (3.18 OR, 95%CI 1.32; 7.68, p = 0.01), fever onset with hospitalized patient (5.88 OR, 95%CI 2.75; 12.57, p < 0.001), first episode of febrile neutropenia (0.34 OR, 95%CI 0.13; 0.92, p = 0.034), and first blood culture with a negative result (0.27 OR, 95%CI 0.07; 0.84, p = 0.025) (Table 4).
Predictive factors for infection in pediatric cancer patients with febrile neutropenia in a reference pediatric unit from June 2016 to June 2018: multivariate analysis.
The p-value is related to the Wald test of Logistic Regression. Pseudo-R2 (Cox & Snell) = 0.239; Pseudo R2 (Nagelkerke) = 0.321.
The model accuracy showed an 84.4% specificity, a 63.2% sensitivity, 25% false positives and 24.3% false negatives. The parameters that evaluated the fit of the logistic model show pseudo-R² values close to, or below, 0.30 (Cox & Snell pseudo-R² = 0.239 and Nagelkerke pseudo-R² = 0.321).
DiscussionThe identification of an infectious focus, defined as documented infection, is limited due to a reduced inflammation response caused by chemotherapy or cancer.17 In this study, 58% of the episodes did not have an identifiable infectious focus, similar to other studies.11,18,19 Bakhshi et al.19 proposed that the absence of clinical signs and symptoms on admission might indicate a mild infection and, presumably, a viral or noninfectious inflammation.
Signs and symptoms of upper respiratory infection, such as cough, runny nose, sneezing and odynophagia were found in 13 episodes and classified as other infections in our descriptive analysis. Rondinelli et al.8 reported that the absence of flu signs at admission is an independent risk factor for severe infection (5.1 OR; 95% CI 1.7 - 15.0, p < 0.001). Such characteristics could influence the decision to authorize early discharge after the first observation.11 To corroborate the clinical impression related to possible viral infections, it would be interesting to perform viral identification tests, however, such tests are not available at the institution under analysis.
Bloodstream infection was the most prevalent documented infection (15.6%), and over half (52%) of the identified pathogens were gram-negative bacteria.
In contrast with studies conducted in developed countries, where gram-positive bacteria are more frequent than gram-negative, the cohort in this study shows similar frequencies among these pathogens. This may be justified by the lower use of long-term catheters, compared to the developed countries, making the pathogens of the gastrointestinal tract still a major cause of bacteremia. A study conducted in El Salvador,20 a developing country similar to Brazil, showed higher rates of gram-positive bacteria than gram-negative, despite the low frequency of central catheters, which highlights the importance of creating a local database which best defines the microbiological profile of each institution.
Although patients with chemotherapy-induced febrile neutropenia have mucosal injury, only 22 cases (10.4%) have been reported with MBI-LCBI because the CDC adheres to a 7-day neutropenia period and defined gastrointestinal tract bacteria. For this reason, not all episodes were classified as MBI-LCBI. We did not find any study in the literature that related bloodstream infection to the breakdown of the mucous barrier.
The five factors independently associated with the infection outcome that remained in the model were also observed by other authors in previous studies.6,21-25 The variable with the highest statistical significance was fever onset during hospitalization. It increased the chance of documented infection by 5.88 times, compared to patients who had fever onset at home. Similarly, Rosenblum et al.21 demonstrated that neutropenic patients hospitalized for more than 48 hours were more likely to have positive blood cultures throughout treatment (OR 3.38 95% CI 1.45; 7.87). Inpatients are exposed to multiresistant pathogens and have a higher risk of infectious complications due to the manipulation of invasive devices and contact with other patients, which highlights the importance of identifying patients who can safely receive early discharge and outpatient treatment.
The second statistically significant factor was the presence of CRP > 90 mg/L at the onset of the episode (OR 3.18 95% CI 1.32; 7.68). A high level of this acute phase reagent is a known risk factor in the literature.6,22-25Santolaya et al.22 established a cutoff point of 90 mg/L as a predictor of severe bacterial infection in conjunction with four additional parameters: hypotension (RR 2.7 95% CI 2.3; 3.2), leukemia relapse (RR 1.8 95% CI 1.7; 2.3), last chemotherapy less than 7 days previously (RR 1.3 95% CI 1.1; 1.6) and platelet count < 50,000/mm³ (RR 1.7 95% CI 1.4; 2.2). In the present study, we chose the same cutoff points for CRP and platelets, as these values have been reproduced over the years in other studies.23,25,26 Although the CRP > 90 mg/L at the onset of the episode has shown an increased risk for infection, it would be interesting to analyze the dynamics of this acute-phase reagent throughout the episode of neutropenia through the CRP curve in future studies.
A platelet count < 50,000/mm³ increases the risk of documented infection by 2.32 times. This variable is an indirect risk factor, as it indicates myelosuppression and possibly a deeper neutropenia with a higher propensity for infectious complications.10,12,22
Two factors were negatively associated with the documented infection in the multivariate analysis. The first episode of febrile neutropenia had reduced the chances of infectious foci being identified, probably because of less exposure to virulent hospital pathogens and less handling of invasive devices, which may have meant a lower risk for unfavorable evolution. Wicki et al.27 showed that previous episodes of febrile neutropenia constitute an independent risk factor for bacteremia in a new episode (RR 2.10 95% CI 1.68 - 2.63 p < 0.001). These results highlight the need for a more rigorous clinical follow-up in patients with more than 1 episode of febrile neutropenia during the chemotherapy treatment.
In the present study, patients whose first blood culture from the febrile episode tested negative for a pathogen were less likely to have documented infection during the neutropenia episode. Hakim et al.11 reported that 93% of the bacteremia was diagnosed within the first 24 hours of febrile neutropenia, with a mean positive time of 12 hours. These results corroborate the US guidelines for pediatric febrile neutropenia, recommending discontinuation of antibiotic therapy in clinically well patients with negative blood cultures at 48 hours, no fever for 24 hours and evidence of bone marrow recovery².
From the accuracy indexes (specificity = 84.4% and sensitivity = 63.7%), the model proves to be useful for identifying children on admission with a low risk for documented infection and moderate ability to predict children at risk for the proposed outcome.
ConclusionThe development of risk stratification scores has become an integral part of the management of FN, as it identifies patients eligible for treatments, resulting in improved quality of life and reduced costs. The present study found 5 factors related to documented infection: CRP > 90 at the onset of the febrile episode, platelet count < 50,000, second episode of febrile neutropenia or more, first fever spike during hospitalization and positive first blood culture, which could be useful in the establishments of risk scores. It is important to highlight that the identified risk factors are accessible clinical and laboratory parameters present in day-to-day medical practice.
Ethics approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Research Ethics Committee of Federal University of Minas Gerais (CAAE – 52792715.5.0000.5149).