Leg ulcers (LUs) are relatively common in patients with sickle cell anemia (SCA). The role of inflammation and nitric oxide (NO) pathways in the pathophysiology of the LU is not understood.
ObjectiveThe aim of this study was to verify the association between inflammatory molecules and nitric oxide metabolites (NOx) and the occurrence of the LU in patients with SCA.
MethodIt was a cross-sectional study on adult participants with SCA followed at Fundação Hemominas, a public blood center in Brazil. Eligible participants were recruited and included in one of two groups: Group 1, comprised of cases with SCA (Hb SS) and at least one LU at the time of inclusion in the study and Group 2, comprised of controls with SCA without a history of LU, matched by sex and age to cases. Participants were interviewed to obtain sociodemographic data and blood samples were collected. Clinical and laboratory data were abstracted from medical records. Nitric oxide metabolites (NOx) and inflammatory molecules were quantified using an immunoassay and Multiplex xMAP® technology, respectively. Eighty-seven individuals were included, ranging in age from 17 to 61 years (mean 40 ± 10.7 years); 30 had LU and 57 were controls without LU.
ResultsParticipants with LU had significantly higher levels of interleukin 8 (IL-8), IL-10, IL-15, NOx and platelet and white blood cell (WBC) counts, when compared to those without LU. Participants with LU had a significantly higher risk of having a history of osteomyelitis and a higher use of antiseptic soap in bathing, when compared to those without LU.
ConclusionIn conclusion, our results showed that NOx, inflammatory molecules and hematological features were associated with LU in Brazilian adults with SCA.
Sickle cell disease (SCD) is a group of genetic disorders caused by a point mutation in the HBB gene (rs334), which encodes the beta-globin chain of hemoglobin (Hb). The pathophysiology is characterized by vaso-occlusive episodes (VOEs) and hemolytic anemia, resulting in both acute complications and progressive organ damage. Although recent advances in treatment strategies have led to considerable progress in the life expectancy of patients with SCD, managing the chronic clinical complications has become an important challenge.1,2
The leg ulcer (LU) is a frequent chronic complication in adult patients with SCD, recognized to be painful, disabling, and difficult to treat. Approximately 2.5–30% of individuals with SCD develop LU during adulthood, varying according to the geographic location.3–7 The pathophysiology of LU in individuals with SCD is complex and unclear. Classical risk factors for the development of LU include increasing age, the homozygous genotype [Hb SS; sickle cell anemia (SCA)], increased level of hemolytic markers and absence of coexisting alpha-thalassemia.7,8 Other environmental and genetic factors also play a role in the development of LU, including poverty4 and a stop codon variant (rs12568784) in the FLG2 gene.9 The LU is a risk factor for early mortality in patients with SCD.8 Therefore, identifying individuals with SCD at a higher risk for LU is important to establish potentially preventive therapy.
The nitric oxide (NO) pathway and inflammation are associated with clinical manifestations of SCD. Sickling and subsequent hemolysis leads to the release of free heme that binds to NO, causing its depletion, consequently resulting in vasoconstriction and inflammation.10,11 The NO bioavailability, vaso-occlusion and impaired oxygen delivery to tissues are involved in the LU pathophysiology.12 Thus, inflammatory markers and NO bioavailability may predict the clinical risk of developing the LU. Only a few studies have explored the role of inflammation and the NO pathway in the development of the LU in patients with SCD, but it is crucial to understand their role in the occurrence and progression of the LU to enable preventive healthcare and identify potential therapeutic targets. In this study, we evaluated the association between the levels of inflammatory molecules, nitric oxide metabolites (NOx) and hematological features with the LU occurrence in individuals with SCA. In addition, we described the LU profile in the study participants.
MethodsStudy design, setting and participantsThis cross-sectional study was conducted at Fundação Hemominas, a comprehensive sickle cell center and publicc blood bank in Belo Horizonte, Minas Gerais, Brazil. Individuals with LU receiving care at the outpatient unit were recruited from 2017 to 2019.
Sickle cell anemia patients without LU receiving treatment at the center were invited to participate as control subjects for the comparison of sociodemographic and laboratory characteristics. Participants with LU were age- (± 3 years) and sex-matched with those without LU. The 1:2 case-to-control ratio was used to increase statistical power and was compatible with the project budget and deadlines.
Eligibility criteriaInclusion criteria for participants with LU were as follows: 1) diagnosis of SCA (HbSS); 2) presence of at least 1 LU at the time of inclusion in the study, and; 3) outpatient follow-up at Fundação Hemominas comprehensive sickle cell center in Belo Horizonte. Inclusion criteria for participants without LU were as follows: 1) diagnosis of SCA (HbSS); 2) outpatient follow-up at Fundação Hemominas; 3) absence of LU at the time of enrollment and absence of a clinical history of LU, and; 4) meeting the criteria of age and sex.
Ethical reviewThe study protocol was approved by the Institutional Review Board of Fundação Hemominas (reference number: 51185415.0.0000.5118). Informed written consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki guidelines.
MeasurementsData collection instrumentsA structured interview, medical record data abstraction and blood collection were performed for all consenting subjects at the time of enrollment. The interview captured sociodemographic information and characteristics of the LU. The LUs were classified according to the Revised Pressure Injury Staging System.13 Medical records were reviewed by researchers and clinical, laboratory and treatment data were abstracted. The blood count used in the analyses was performed within 3 months before study enrollment. For 8 participants who did not have a steady-state blood count within 3 months, the closest blood count test results performed to the study enrollment, within 3 and 8 months were considered. There was no change in the participant treatment protocol between performing the blood count used in the analysis and the study enrollment. Blood count data were abstracted only if measured in participant steady-state, that is, in routine clinical visits at least 2 months without of an acute event.
The HbSS was diagnosed as part of routine clinical care, which typically involves laboratory (blood count, high performance liquid chromatography and hemoglobin electrophoresis) and clinical evaluation and the acquisition of the family history. For participants born after 1998, the diagnosis also includes newborn screening data.
Laboratory methodsThe total Hb, mean corpuscular volume and the white blood cell (WBC), platelet and reticulocyte counts were determined using an automated cell counter (CELL-DYN Ruby, Abbott Laboratories, Santa Clara, CA, USA). The quantification of the Hb fractions was performed by high-pressure liquid chromatography (HPLC), using the Beta-thalassemia Short Program on the Variant II Analyzer (BioRad, Hercules, CA, USA).
Blood samples were collected in heparin tubes from steady-state (as previously defined) participants with SCA, immediately centrifuged at room temperature, transferring the plasma fraction to 2 cryotubes that were stored at −80 °C. Multiple inflammatory molecules were assessed in plasma samples using Bio-Plex Pro™ Human Cytokine 27-plex Assay (Biorad, Hercules, CA, USA). The following inflammatory molecules were assessed: CXCL8 (interleukin 8, or IL-8), CXCL10 (human interferon-inducible protein, or IP-10), CCL11 (eotaxin), CCL3 (MIP-1α), CCL4 (MIP1β), CCL2 (MCP-1), CCL5 (RANTES), IL-1β, IL-6, TNF-α, IL-12, IFN-g, IL-17, IL-1Ra (IL-1 receptor antagonist), IL-2, IL-4, IL-5, IL-7, IL-9, IL-10, IL-13, IL-15, basic fibroblast growth factor (FGF-basic), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF). The assessments were performed according to the manufacturer’s instructions. Acquisition was performed using a Bio-Plex® 200 system (Biorad, Hercules, CA). Data were acquired with Bio-Plex Systems 100 (Biorad, CA, USA) and analyzed using the Bio-Plex Manager v6.2 software. The levels of IL-12, IL-5, IL-7, VEGF and GM-CSF were below the detection limits in several samples and therefore, were excluded from further analysis. Nitric oxide metabolites were measured using the Nitric Oxide Assay Kit (catalog number: EMSNO, ThermoFisher Scientific, Carlsbad, CA, USA). The nitrate reductase enzyme converts nitrate to nitrite, which is detected as a colored azo dye product of the Griess reaction that absorbs visible light at 540 nm. The interaction of NO in a system is measured by determining of both nitrate and nitrite concentrations in the sample. The procedures were performed according to the manufacturer’s instructions. The sensitivity of the assays was 0.222 µM for nitrite and 0.625 µM for nitrate.
Statistical analysisOur principal outcome of interest was the LU. The independent variables were laboratory data. Continuous variables are presented as mean and standard deviation or median and interquartile range (IQR), as appropriate. Categorical variables are expressed as percentages of the total. Normal distribution of continuous variables was verified by the Shapiro-Wilk test. Analysis of continuous variables with normal distribution was performed using the unpaired t-test; continuous variables without normal distribution were analyzed using the Mann–Whitney U test. Associations between categorical variables were evaluated using the two-tailed Chi-square or Fisher exact test. Pearson or Spearman correlation analysis examined the relationship between two continuous variables. Binary logistic regression was used to adjust the effect of confounders (therapies in use) and determine the independent effect of each variable on the outcome. Tests were considered significant when the probability of alpha error was < 0.05. Statistical analyses were performed with the SPSS 23.0 (SPSS Inc.; Chicago, IL, USA) or GraphPad Prism 6.0 (La Jolla, California) software.
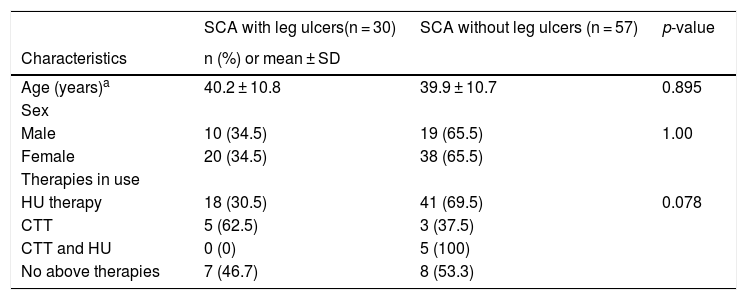
ResultsParticipant descriptionThe study population comprised 87 individuals (58 women; 66.7%) with a mean age of 40 ± 10.7 years (17–61 years). Of these participants, 50 (67.8%) were receiving hydroxyurea (HU) therapy, 8 (9.2%) were receiving chronic transfusion therapy (CTT), 5 (5.7%) were receiving both HU and CTT and 15 (17.2%) were not receiving either of the therapies at the time of the study enrollment. The mean dose of HU was 18.8 ± 4.6 mg/kg/day, for a mean duration of 43.5 ± 40.2 months. All participants were homozygous HbSS. Participants with and without LU were similar, with respect to age and sex (Table 1).
Characteristics of the 87 participants enrolled in the study to evaluate the association of demographic and laboratory characteristics with leg ulcers at the Hemocenter of Belo Horizonte, Fundação Hemominas.
| SCA with leg ulcers(n = 30) | SCA without leg ulcers (n = 57) | p-value | |
|---|---|---|---|
| Characteristics | n (%) or mean ± SD | ||
| Age (years)a | 40.2 ± 10.8 | 39.9 ± 10.7 | 0.895 |
| Sex | |||
| Male | 10 (34.5) | 19 (65.5) | 1.00 |
| Female | 20 (34.5) | 38 (65.5) | |
| Therapies in use | |||
| HU therapy | 18 (30.5) | 41 (69.5) | 0.078 |
| CTT | 5 (62.5) | 3 (37.5) | |
| CTT and HU | 0 (0) | 5 (100) | |
| No above therapies | 7 (46.7) | 8 (53.3) | |
HU: hydroxyurea; CTT: chronic transfusion therapy.
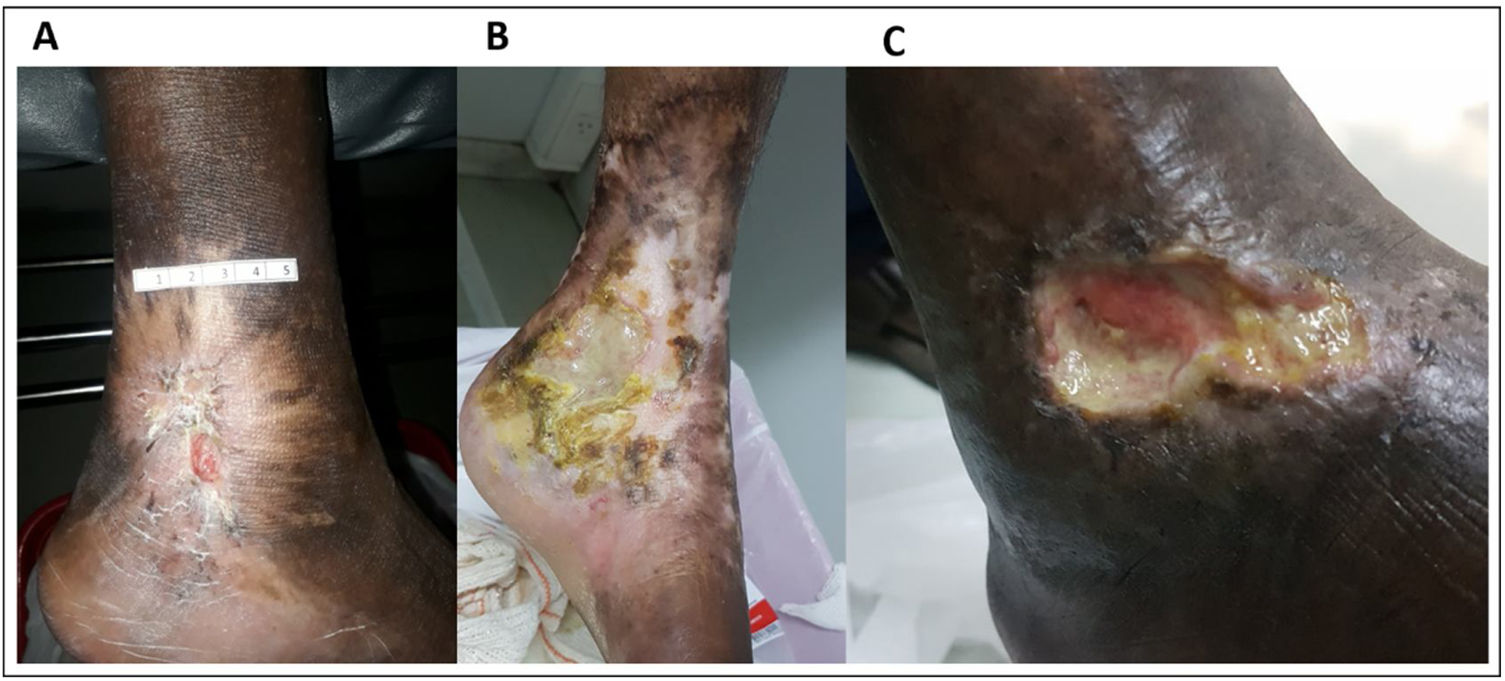
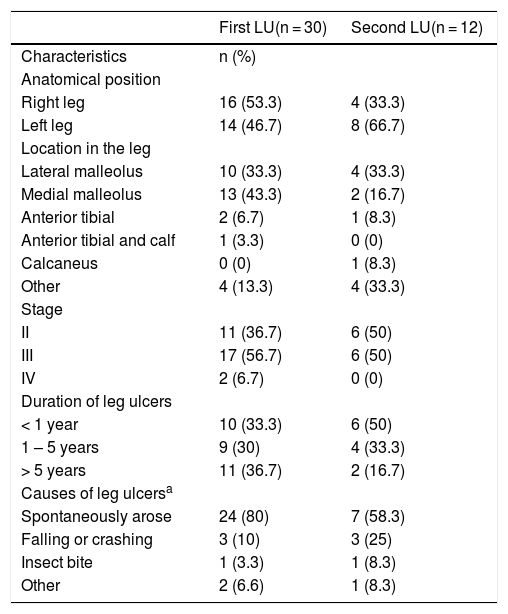
At the time of the study enrollment, 60% (n = 18/30) of the participants with LU had 1 ulceration and 40% (n = 12/30) had 2. Characteristics, severity and duration of LU varied among participants with SCA (Table 2 and Fig. 1). All (n = 30; 100%) participants reported pain related to LU; 56.7% (n = 17/30) reported use of medication to treat the LU in the past 12 months; most patients reported use of ciprofloxacin (29.5%; n = 5), followed by hydrogel (17.6%; n = 3). Additionally, 30% (n = 9/30) participants reported the use of homemade herbal medicine in wound care; 77.8% (n = 7/9) reported the use of tea, 1 (11.1%) reported the use of sugar and 1 (11.1%) reported the use of a combination of saffron and olive oil.
Characteristics of leg ulcers in the 30 participants with SCA enrolled in the study.
| First LU(n = 30) | Second LU(n = 12) | |
|---|---|---|
| Characteristics | n (%) | |
| Anatomical position | ||
| Right leg | 16 (53.3) | 4 (33.3) |
| Left leg | 14 (46.7) | 8 (66.7) |
| Location in the leg | ||
| Lateral malleolus | 10 (33.3) | 4 (33.3) |
| Medial malleolus | 13 (43.3) | 2 (16.7) |
| Anterior tibial | 2 (6.7) | 1 (8.3) |
| Anterior tibial and calf | 1 (3.3) | 0 (0) |
| Calcaneus | 0 (0) | 1 (8.3) |
| Other | 4 (13.3) | 4 (33.3) |
| Stage | ||
| II | 11 (36.7) | 6 (50) |
| III | 17 (56.7) | 6 (50) |
| IV | 2 (6.7) | 0 (0) |
| Duration of leg ulcers | ||
| < 1 year | 10 (33.3) | 6 (50) |
| 1 – 5 years | 9 (30) | 4 (33.3) |
| > 5 years | 11 (36.7) | 2 (16.7) |
| Causes of leg ulcersa | ||
| Spontaneously arose | 24 (80) | 7 (58.3) |
| Falling or crashing | 3 (10) | 3 (25) |
| Insect bite | 1 (3.3) | 1 (8.3) |
| Other | 2 (6.6) | 1 (8.3) |
LU: leg ulcer.
Participants with LU reported a significantly higher use of an antiseptic soap in bathing (OR = 6.47; 95% CI, 1.79–23.45; p = 0.004) than those without LU. Participants with LU had a significantly higher risk of a history of osteomyelitis than those without LU (OR = 6.88; 95% CI, 1.29–36.54; p = 0.018).
Association of LUs with laboratory dataThe WBC and platelet counts were significantly higher in participants with LU than in those without LU (Table 3). The platelet and WBC counts were significantly higher in participants receiving CTT therapy than in those receiving HU therapy (p = 0.006 and 0.003, respectively). The association between the platelet and WBC counts with LU remained significant after adjusting for therapy in use (p = 0.005 and 0.002, respectively).
Association between laboratory data and leg ulcers among 87 participants with sickle cell anemia.
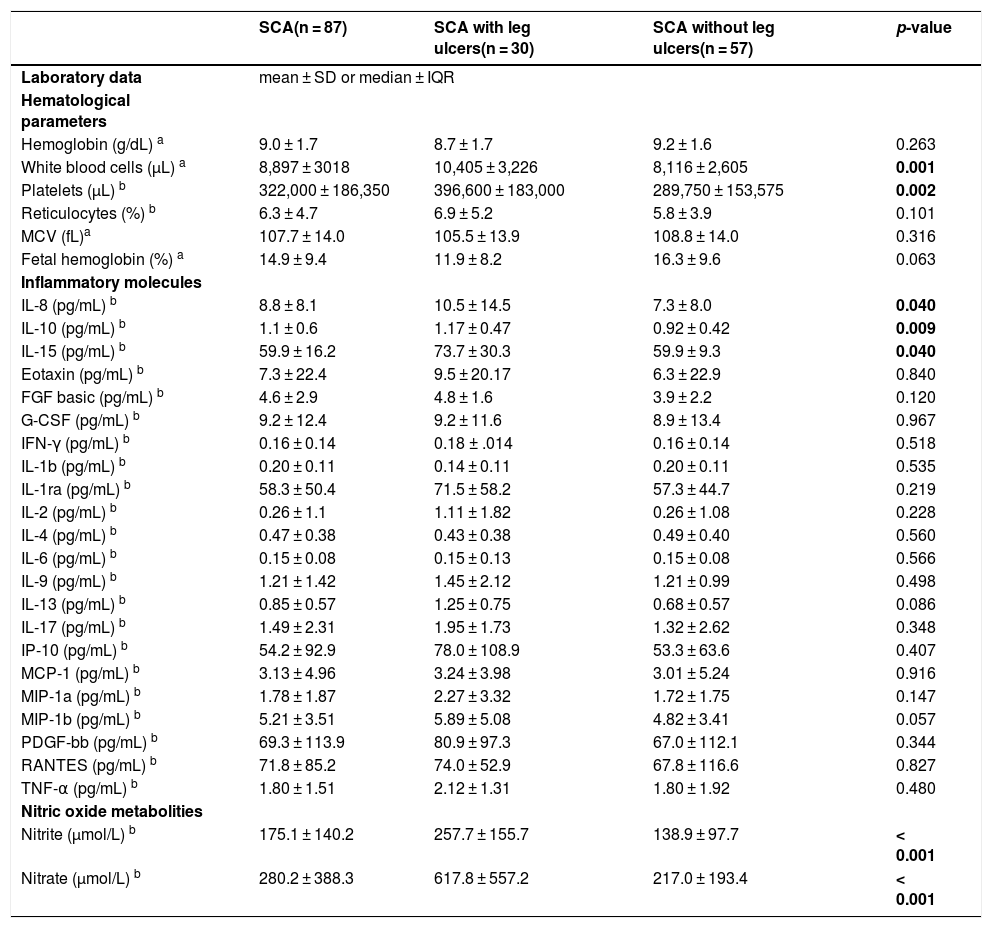
| SCA(n = 87) | SCA with leg ulcers(n = 30) | SCA without leg ulcers(n = 57) | p-value | |
|---|---|---|---|---|
| Laboratory data | mean ± SD or median ± IQR | |||
| Hematological parameters | ||||
| Hemoglobin (g/dL) a | 9.0 ± 1.7 | 8.7 ± 1.7 | 9.2 ± 1.6 | 0.263 |
| White blood cells (µL) a | 8,897 ± 3018 | 10,405 ± 3,226 | 8,116 ± 2,605 | 0.001 |
| Platelets (µL) b | 322,000 ± 186,350 | 396,600 ± 183,000 | 289,750 ± 153,575 | 0.002 |
| Reticulocytes (%) b | 6.3 ± 4.7 | 6.9 ± 5.2 | 5.8 ± 3.9 | 0.101 |
| MCV (fL)a | 107.7 ± 14.0 | 105.5 ± 13.9 | 108.8 ± 14.0 | 0.316 |
| Fetal hemoglobin (%) a | 14.9 ± 9.4 | 11.9 ± 8.2 | 16.3 ± 9.6 | 0.063 |
| Inflammatory molecules | ||||
| IL-8 (pg/mL) b | 8.8 ± 8.1 | 10.5 ± 14.5 | 7.3 ± 8.0 | 0.040 |
| IL-10 (pg/mL) b | 1.1 ± 0.6 | 1.17 ± 0.47 | 0.92 ± 0.42 | 0.009 |
| IL-15 (pg/mL) b | 59.9 ± 16.2 | 73.7 ± 30.3 | 59.9 ± 9.3 | 0.040 |
| Eotaxin (pg/mL) b | 7.3 ± 22.4 | 9.5 ± 20.17 | 6.3 ± 22.9 | 0.840 |
| FGF basic (pg/mL) b | 4.6 ± 2.9 | 4.8 ± 1.6 | 3.9 ± 2.2 | 0.120 |
| G-CSF (pg/mL) b | 9.2 ± 12.4 | 9.2 ± 11.6 | 8.9 ± 13.4 | 0.967 |
| IFN-γ (pg/mL) b | 0.16 ± 0.14 | 0.18 ± .014 | 0.16 ± 0.14 | 0.518 |
| IL-1b (pg/mL) b | 0.20 ± 0.11 | 0.14 ± 0.11 | 0.20 ± 0.11 | 0.535 |
| IL-1ra (pg/mL) b | 58.3 ± 50.4 | 71.5 ± 58.2 | 57.3 ± 44.7 | 0.219 |
| IL-2 (pg/mL) b | 0.26 ± 1.1 | 1.11 ± 1.82 | 0.26 ± 1.08 | 0.228 |
| IL-4 (pg/mL) b | 0.47 ± 0.38 | 0.43 ± 0.38 | 0.49 ± 0.40 | 0.560 |
| IL-6 (pg/mL) b | 0.15 ± 0.08 | 0.15 ± 0.13 | 0.15 ± 0.08 | 0.566 |
| IL-9 (pg/mL) b | 1.21 ± 1.42 | 1.45 ± 2.12 | 1.21 ± 0.99 | 0.498 |
| IL-13 (pg/mL) b | 0.85 ± 0.57 | 1.25 ± 0.75 | 0.68 ± 0.57 | 0.086 |
| IL-17 (pg/mL) b | 1.49 ± 2.31 | 1.95 ± 1.73 | 1.32 ± 2.62 | 0.348 |
| IP-10 (pg/mL) b | 54.2 ± 92.9 | 78.0 ± 108.9 | 53.3 ± 63.6 | 0.407 |
| MCP-1 (pg/mL) b | 3.13 ± 4.96 | 3.24 ± 3.98 | 3.01 ± 5.24 | 0.916 |
| MIP-1a (pg/mL) b | 1.78 ± 1.87 | 2.27 ± 3.32 | 1.72 ± 1.75 | 0.147 |
| MIP-1b (pg/mL) b | 5.21 ± 3.51 | 5.89 ± 5.08 | 4.82 ± 3.41 | 0.057 |
| PDGF-bb (pg/mL) b | 69.3 ± 113.9 | 80.9 ± 97.3 | 67.0 ± 112.1 | 0.344 |
| RANTES (pg/mL) b | 71.8 ± 85.2 | 74.0 ± 52.9 | 67.8 ± 116.6 | 0.827 |
| TNF-α (pg/mL) b | 1.80 ± 1.51 | 2.12 ± 1.31 | 1.80 ± 1.92 | 0.480 |
| Nitric oxide metabolities | ||||
| Nitrite (µmol/L) b | 175.1 ± 140.2 | 257.7 ± 155.7 | 138.9 ± 97.7 | < 0.001 |
| Nitrate (µmol/L) b | 280.2 ± 388.3 | 617.8 ± 557.2 | 217.0 ± 193.4 | < 0.001 |
MCV: mean corpuscular volume; SD: standard deviation; IQR: interquartile range; IL: interleukin; FGF basic: basic fibroblast growth factor; G-CSF: granulocyte colony-stimulating factor; IFN-γ: interferon-gamma; IP-10: interferon-γ-inducible protein-10; MCP-1: monocyte chemotactic protein-1; MIP-1: macrophage inflammatory protein 1; PDGF: platelet-derived growth factor; TNF-α: tumor necrosis factor alpha.
Bold values denote statistical significance at the p < 0.05 level.
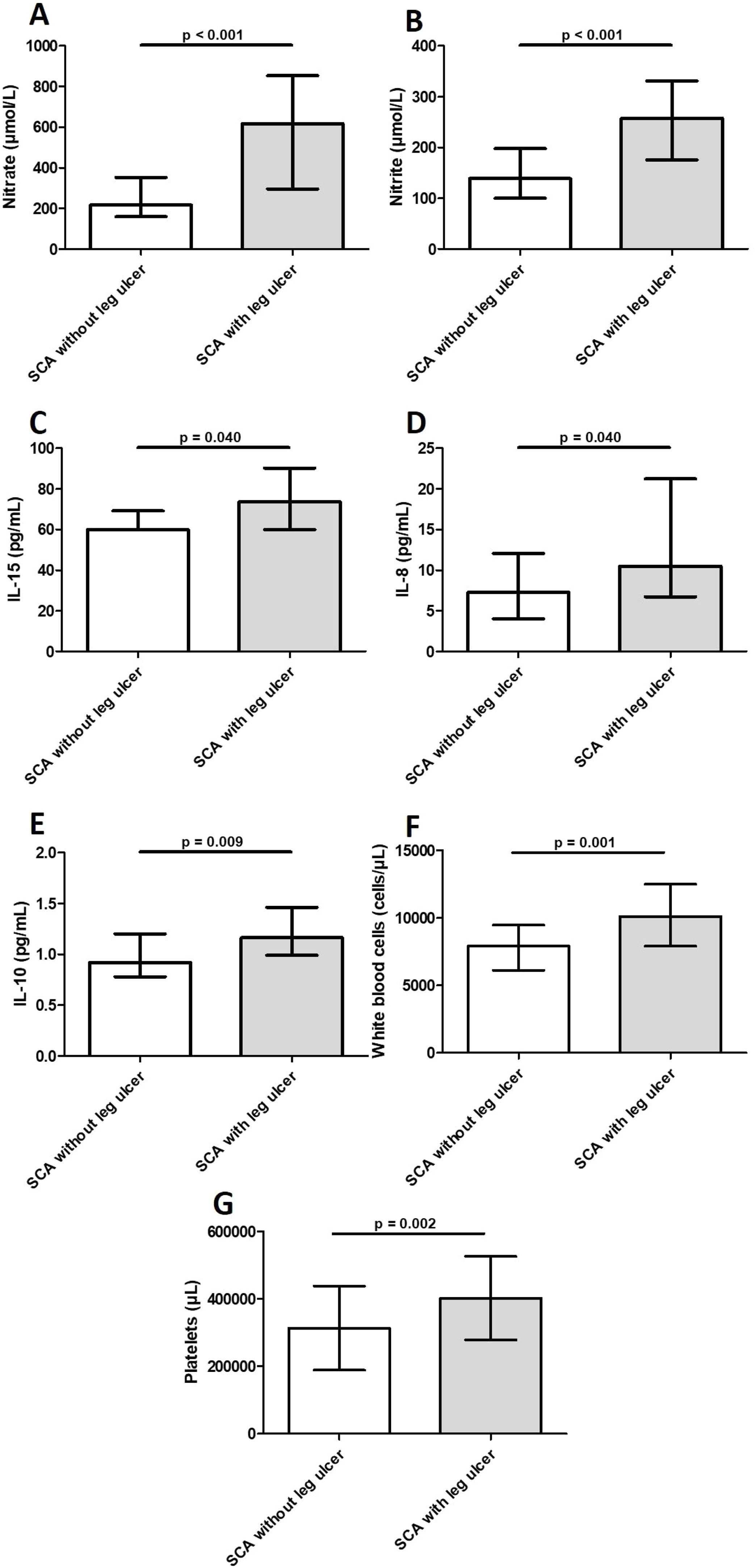
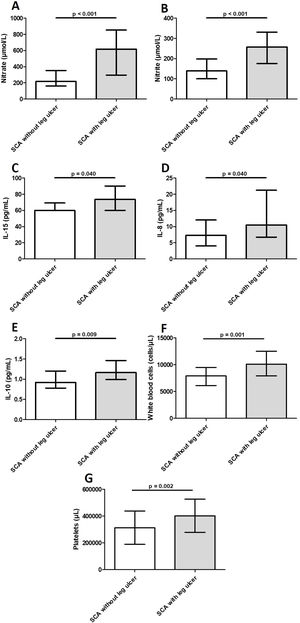
Levels of IL-8, IL-10, and IL-15 were significantly higher in participants with LU than in those without LU (Table 3 and Fig. 2). Additional analysis using the median value revealed 3.5 times (OR = 3.51; 95% CI, 1.27–9.69; p = 0.017) greater odds of participants with IL-10 above the median having LU. Similarly, participants with IL-8 above the median had 2.9 times (OR = 2.86; 95% CI, 1.06–7.78; p = 0.05) greater odds of having LU. The median PDGF levels were significantly higher in the group of participants with LU for > 5 years (123.2 ± 91.3) than in those with LU for ≤ 5 years (66.1 ± 81.1) (p = 0.046). Levels of FGF-basic were significantly higher in the group of participants with 2 LUs (5.5 ± 2.1) than in those with 1 LU (4.4 ± 2.4) (p = 0.039). There was no association between levels of inflammatory molecules and LU stage (data not shown).
Laboratory markers levels in blood samples from individuals with sickle cell anemia, with and without leg ulcers. Individuals with leg ulcers presented significantly increased levels of nitrate (2A), nitrite (2B), IL-15 (2C), IL-8 (2D), IL-10 (2E), WBC (2F) and platelets (2G). Horizontal bars in each figure show median and interquartile ranges (25th percentile - 75th percentile). *Statistically significant p-values. Legend: IL, interleukin; WBCs, white blood cells.
Levels of NOx were significantly higher in participants with LU than in those without LU (Table 3 and Fig. 2). Participants with nitrite and nitrate above the median had 6.3 (OR: 6.3; 95% CI, 2.16–18.20; p = 0.001) and 7.4 (OR: 7.4; 95% CI, 2.53–21.71; p < 0.001) times greater odds of having LU, respectively. The NOx levels were higher in the group of participants with LU in stages 3 or 4 than in those with LU in stage 2, but the difference was not significant (data not shown). Participants with nitrate above the median had 16 times (OR: 16.0; 95% CI, 1.49–171.20; p = 0.015) greater odds of having LU in stages 3 or 4. There was no association between NOx levels and LU duration or the number of LUs (data not shown).
Among the variables that were significantly associated with the LU, levels of nitrate strongly correlated positively with nitrite levels (rho = 0.90, p < 0.001). In addition, the IL-15 level was significantly correlated with the platelet count (rho = 0.343, p = 0.021) and the WBC count was significantly correlated with the platelet count (rho = 0.490, p < 0.001).
DiscussionThis is one of the few studies that evaluated the association of inflammatory molecules and NOx levels with LU occurrence in individuals with SCA. In our study, we found that both NOx and inflammatory molecules were associated with LU. Furthermore, we confirmed that the LU is related to some laboratory markers.
The NO plays a critical role in the pathophysiology of SCD10 and is a promising biomarker and therapeutic target. In our study, we found a significant association between higher NOx levels and the LU. A previous study also suggested the involvement of the NO pathway in the LU, although lower levels of NOx were found in patients with LU than in control subjects.14 Higher levels of NOx were previously associated with worse laboratory severity in Brazilian patients with SCD, including an increased reticulocyte count.15 Production of NO is increased in wound tissue due to the activation of endothelial nitric oxide synthase and increased production of inducible nitric oxide synthase.16 In a study using an SCD mouse model, endothelial nitric oxide synthase-deficient SCD animals presented reduced platelets and WBC adhesion, whereas nitric oxide synthase-overexpressing animals presented overstated adhesion responses, leading to an inflammatory and prothrombogenic environment in the microvasculature.17 Interestingly, our data revealed significantly higher levels of both WBC and platelet levels in participants with LU. It can also be hypothesized that the higher NOx levels observed in participants with LU are a consequence of a higher requirement for NO to neutralize inflammation, promote vasodilatation, inhibit platelet aggregation, and activate endothelial cells. Our results, revealing significantly higher nitrate levels in participants with more severe LU (stages 3 or 4), support this hypothesis. Alternatively, the association between increased NOx levels and LU vasculopathy may reflect a consumption of NO by plasma cell-free hemoglobin due to severe hemolysis, generating a state of NO resistance characterized by compromised NO bioavailability. This state has been associated with an impaired blood flow physiology and a higher prevalence of LU in patients with SCD.18 Additional studies are needed to better define the mechanism of the association between NO levels and the LU in individuals with SCA.
Some studies suggest the involvement of hemolytic markers in the occurrence of the LU,7,8 although there are no studies indicating that hypercoagulability and inflammation play a role in the physiopathology of the LU in individuals with SCA. In our study, the platelet and WBC counts were significantly higher in patients with LU. Platelets play an important role in the VOEs, forming aggregates with WBCs and secreting inflammatory cytokines and chemokines.19 Our data indicated that hypercoagulability probably intensifies inflammation in individuals with LU, as the measured levels of platelets were positively correlated with the WBC count and IL-15 level. The IL-15 is a proinflammatory cytokine that plays a central role in the defense mechanisms against pathogens20 and in the tissue-resident memory T-cell homeostasis in the epidermis,21 which may explain the association with the LU found in our study. In turn, IL-8 and IL-10 are important inflammatory molecules and their levels have been reported to be increased in patients with SCA during the VOE22 and albuminuria.23 Recently, Domingos et al. demonstrated an association between increased IL-8 levels and LU occurrence in individuals with SCA followed up at a northeastern Brazilian reference center.24 It has been suggested that the IL-8 acts as an activator for the epithelial to mesenchymal transition in the wound healing of diabetic foot ulcers,25 in addition to its principal role as a chemokine for neutrophils. The increased level of IL-8 in participants with LU in our study may represent the activation of wound healing. The IL-10 is an anti-inflammatory molecule that might increase the odds of LU, due to the suppression of several proinflammatory cytokines. However, the IL-10 inhibits the immune response from WBCs and restricts the possible tissue injury caused by inflammation.26 Additional studies are required to clarify the role of inflammation in the pathophysiology of the LU in patients with SCD.
Our data suggest that the inflammatory immune response is modulated by the severity of the LU because increased FGF-basic and PDGF levels were observed in participants with 2 LUs and those with LUs lasting > 5 years, respectively. These findings indicate a possible compensatory mechanism that individuals with more severe LU presentation might develop to enhance wound healing capacity and promote LU closure. The FGF-basic has been used therapeutically to accelerate wound healing due to its role in angiogenesis and tissue formation.27 The PDGF is an important mitogen that has shown the potential to improve wound healing.28 These data also suggest that the amplification of the inflammatory state in the individuals with SCA is an effect of the LU. Supporting this postulate is the absence of association between WBCs and the occurrence of the LU in previous prospective studies in which the measurement of possible markers was performed before the development of the outcome.3
Our data revealed that individuals with LU had about 7 times higher odds of having a history of osteomyelitis. From these data, it is reasonable to conclude that one bacterial source of contamination affecting the bones of patients with SCD is ulcerations, suggesting that patients with LU should be strictly monitored for the occurrence of osteomyelitis. The risk of infection is a concern in patients with LU. Our study demonstrated that self-care for the LU includes the use of an antiseptic soap, instead of a conventional soap, and the antiseptic soap use is probably a response to having an LU, rather than being a contributor to the LU. Local therapy for managing the LU in patients with SCD involves conventional wound care and interventions, including wearing comfortable footwear.29 However, there is no evidence supporting the current management of the LU in patients with SCD30 and the development of high-quality clinical trials are warranted.
One of the limitations of this study is its single-center, cross-sectional design. Inflammatory molecules and NOx levels were measured in patients with established LU, which increases the possibility that they were the consequence of the complication and not the cause. The causation may only be established in a prospective study with a baseline dosage of NOx or inflammatory molecules before the development of the LU. The NOx and inflammatory pathways have an influence on the occurrence of several clinical manifestations of SCD and the presence of chronic comorbidities, such as kidney disease, osteonecrosis and pulmonary hypertension, may have acted as confounding factors in our analysis. Furthermore, although we have proposed some plausible explanations for the role of NOx and inflammatory molecules in the pathophysiology of LU, the exact mechanism by which these factors act in the development of the LU remains unclear and more experimental studies are required. Additionally, the small sample size may have resulted in a lack of statistical power to detect associations, especially in the analysis restricted to those with LU.
In conclusion, our results revealed that the levels of NOx and inflammatory molecules were associated with the LU in Brazilian adults with SCA. Additionally, LU was associated with the hematological features of SCA. These data provide insights into the pathophysiological mechanism of the LU in individuals with SCA, as well as enable early and accurate identification of at-risk individuals and the use of therapeutic interventions to prevent complications.
FundingThis work was supported by the “Fundação de Amparo à Pesquisa de Minas Gerais” [grant number APQ-01764-16].
Conflicts of interestThe authors declare no conflicts of interest.
The authors acknowledge all participants for their cooperation in the study. The authors also thank the program for technological development in tools for health-FIOCRUZ for the use of Flow Cytometry Platform.