The long-term outcome of acute lymphoblastic leukemia has improved dramatically due to the development of more effective treatment strategies. L-asparaginase (ASNase) is one of the main drugs used and causes death of leukemic cells by systematically depleting the non-essential amino acid asparagine. Three main types of ASNase have been used so far: native ASNase derived from Escherichia coli, an enzyme isolated from Erwinia chrysanthemi and a pegylated form of the native E. coli ASNase, the ASNase PEG. Hypersensitivity reactions are the main complication related to this drug. Although clinical allergies may be important, a major concern is that antibodies produced in response to ASNase may cause rapid inactivation of ASNase, leading to a worse prognosis. This reaction is commonly referred to as "silent hypersensitivity" or "silent inactivation". We are able to analyze hypersensitivity and inactivation processes by the measurement of the ASNase activity. The ability to individualize the ASNase therapy in patients, adjusting the dose or switching patients with silent inactivation to an alternate ASNase preparation may help improve outcomes in those patients. This review article aims to describe the pathophysiology of the inactivation process, how to diagnose it and finally how to manage it.
During the last decades, the long-term outcome of acute lymphoblastic leukemia (ALL) has improved dramatically due to the development of effective treatments and well-designed protocols according to malignant cells origin. Long-term event-free survival in children is currently around 80%, and overall survival is close to, or in excess of, 90% in 5 years in high-income countries.1 Among the drugs used as the cornerstone of combination protocols in the treatment of leukemias is the bacterial enzyme L-asparaginase (ASNase).1,2
In 1953, tumor-inhibitory properties of ASNase were first described by Kidd, with the observation that guinea-pig serum treated lymphoma-bearing mice (particularly 6C3HED) underwent rapid and often complete tumor regression.3,4 These properties were later attributed to the ASNase activity.2,5 In 1963, Mashburn and Wriston found that the E. coli enzyme had anti-tumor activity.6
Although it may be considered an old drug, we are still learning about its mechanism and the necessary care when prescribing it. Actually, pharmacokinetic properties of ASNase are dependent on several different factors, including the bacterial source.7,8 Three main types of ASNase have been used so far: native ASNase derived from Escherichia coli, an enzyme isolated from Erwinia chrysanthemi, referred to as Erwinia ASNase and a pegylated form of the native E. coli ASNase.2,9,10 The enzyme derived from E. coli is indicated in most first-line therapy, while the Erwinia-derived ASNase is reserved for patients who develop hypersensitivity reactions to the previous form.10–12
Since ASNase preparations are derived from bacteria, they are highly immunogenic. For this reason, a third formulation, the PEG ASNase, which is a polyethylene glycol conjugated ASNase, has been developed in order to reduce the immunogenicity, as well as the number of infusions. It is well known that the pegylated form results in reduced rates of antibody formation, a lower incidence of allergy, and a prolonged serum half-life.12 Due to its pharmacological characteristics, it has been used as the initial preparation of ASNase in some ALL treatment regimens.1Actually, the PEG-ASNase has a half-life of about 1 week, while native E. coli ASNase and Erwinia ASNase have a half-life of 1.3 and 0.65 days, respectively.13 Due to the shorter half-life of Erwinia ASNase, a higher dose and frequency of applications are required to ensure adequate serum enzyme activity.14
The administration route of ASNase derived from E. coli can be both intravenous (IV) or intramuscular (IM). However, PEG-ASNase and Erwinia ASNase preferentially have IM administration, a some studies have shown that these medications may present a greater immunogenic potential when IV. It is important to mention that the IV administration is less painful and may be more convenient in specific settings.12,15
ASNase is associated with different adverse reactions, but the major limitation in delivering the intended up-front ASNase therapy is the high rate of hypersensitivity reactions (30%–70% of patients receiving E. coli derived ASNase).16,17 Other side effects are hypoalbuminemia, anaphylaxis, pancreatitis, hyperglycemia, hyperlipidemia, urticaria, bronchospasm, angioedema and coagulation abnormalities that may lead to intracranial thrombosis or hemorrhage.9,10
More recently, in 2004, Panosyan et al. have described that patients with clinical hypersensitivity have a faster clearance when compared to patients who do not have this reaction.16 In addition, antibodies produced in response to ASNase do not always lead to clinical hypersensitivity, but could instead cause rapid inactivation of ASNase, resulting in suboptimal asparagine depletion and sub-therapeutic serum concentrations, leading to decreased survival and a greater chance of the relapse of the disease.10,16
This review article aims to describe the update of the major advances of the pathophysiology, clinical management of ASNAse and its modern clinical application in ALL acquired overtimes.
Pathophysiology of the hypersensitivity and inactivation processUpon further study, it was observed that ASNase causes the death of leukemic cells by systematically depleting the non-essential amino acid asparagine. These cells are particularly sensitive because they have low levels of asparagine-synthetase. The ASNase owes its antileukemic effect to the rapid and almost complete conversion of circulating Asn concentrations to aspartic acid and ammonia. For these reasons, serum Asn deamination selectively eliminates leukemia cells, resulting in reduced protein synthesis and, ultimately, leukemic cell death, preserving normal cells, as the latter have the ability to synthesize it intracellularly.1,2,11
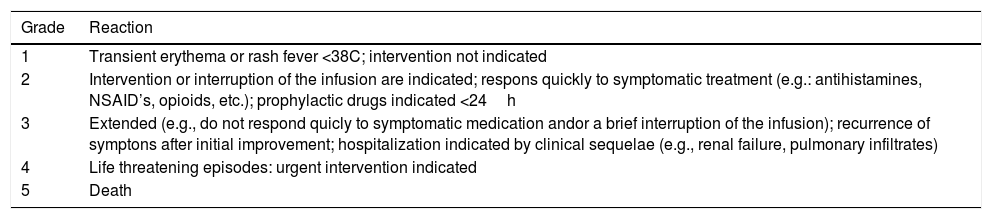
Clinical hypersensitivity is one of the most common reasons for the discontinuation of the ASNase therapy.18 It is characterized by an allergic reaction with signs and symptoms consistent with an immune response to a known antigen.10 Although the specific mechanism responsible for the ASNase-induced hypersensitivity is unknown, most cases manifest a combination of symptoms that can vary from mild to severe.19,20 The severity of the reaction is classified according to the Common Toxicity Criteria for Adverse Events (CTCAE) (Table 1) where mild-to-moderate reactions are characterized by flushing, fever, chills and dyspnea while severe reactions can include bronchospasm and anaphylaxis.21 A number of less prevalent adverse events, including hyperglycemia, vomiting, pancreatitis, nausea, abdominal pain and diarrhea may also occur.10
CTCAE criteria for toxicity in hypersensitivity reactions.
| Grade | Reaction |
|---|---|
| 1 | Transient erythema or rash fever <38C; intervention not indicated |
| 2 | Intervention or interruption of the infusion are indicated; respons quickly to symptomatic treatment (e.g.: antihistamines, NSAID’s, opioids, etc.); prophylactic drugs indicated <24h |
| 3 | Extended (e.g., do not respond quicly to symptomatic medication andor a brief interruption of the infusion); recurrence of symptons after initial improvement; hospitalization indicated by clinical sequelae (e.g., renal failure, pulmonary infiltrates) |
| 4 | Life threatening episodes: urgent intervention indicated |
| 5 | Death |
Source: Barba et al.14.
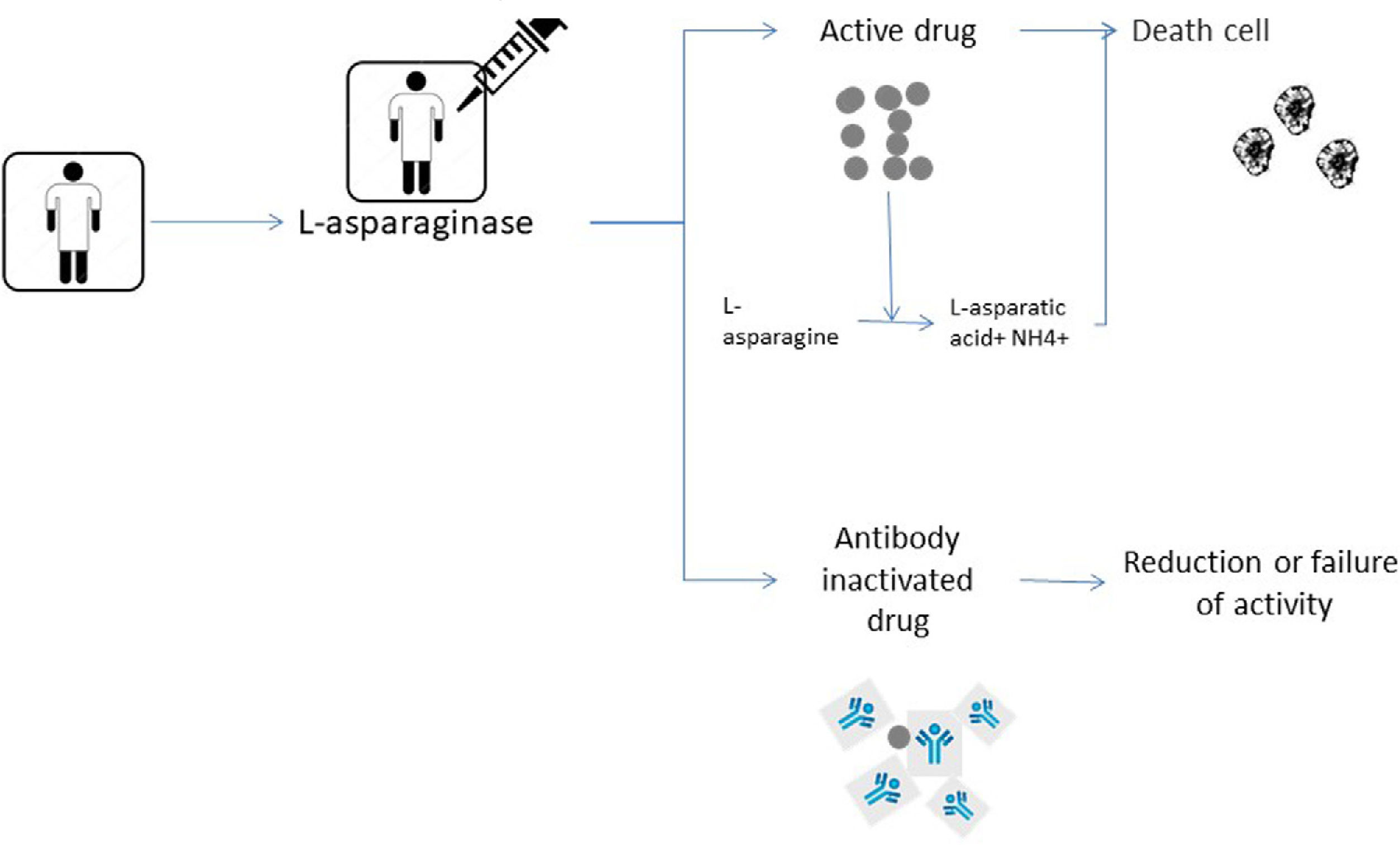
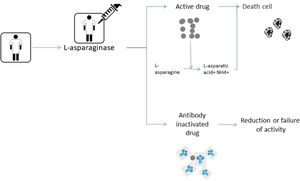
Patients developing a clinical allergy, as well as some patients without any clinical signs of hypersensitivity reactions, can have reduced ASNase activity levels due to the presence of a neutralizing antibody.8,22,23 The development of antiasparaginase neutralizing antibodies in the absence of clinical symptoms has been termed “silent inactivation”.14 The pharmacokinetic and pharmacodynamic consequences of the neutralizing antibodies produced on silent inactivation remain unclear.24 These antibodies alter the pharmacokinetics and bioavailability of the drug.21 Patients who develop hypersensitivity have high titers of immunoglobulin G (IgG) and immunoglobulin E (IgE) antibodies to asparaginase leading to decreased activity, which may represent a mechanism of resistance to chemotherapy.24,25 In fact, ASNase activity levels are inversely correlated with anti-ASNase antibody levels.10,26 Due to this fact, patients who experience a severe hypersensitivity reaction are likely to exhibit significantly reduced ASNase activity and higher serum asparagine levels shortly after dose administration.10Fig. 2 shows the mechanism of action of ASNase in leading to death of the tumor cell and immediately below the process of silent inactivation where it leads to the reduction or failure of ASNase activity due to the antibodies produced.
Shows the pathophysiology of the mechanism of action of ASNase. The figure above shows the normal functioning of ASNase degrading asparagine and leading to cell death. And the figure below shows the silent inactivation situation where antibody production leads to low or loss of ASNase activity.
According to Burke et al., the risk of the development of clinical allergy and silent inactivation may be influenced by several factors, including the formulation preparation, the route of administration, the schedule of administration, the protocol of treatment and the concurrent use of other chemotherapeutic agents, including corticosteroids.10 Hypersensitivity reactions are more likely to appear with increasing numbers of administrations within the same cycle but may appear in discontinuous administration regimens.27 These reactions are more common in the first doses of asparaginase and after a break in treatment. The risk of antibody formation increases with repeated exposure to ASNase, especially in the consolidation and re-induction phases of the treatment. However, prolonged exposure to ASNase, with no treatment gaps, has been associated with low antibody levels.20 Salzer et al. discuss the fact that immunosuppression induced by corticosteroids and concomitant chemotherapy may have prevented the development of antibodies or less synthesis of them.24 However, premedication with steroids or antihistamines is known to reduce allergy symptoms, but may not prevent the development of antibodies.14
Dominiki et al. reported that to estimate the intensity of the ASNase treatment, the ASNase activity (U/mL) serves as a sensitive parameter.11 The activity of ASNase ≥0.1IU/mL is considered to be effective for the complete depletion of asparagine.13,17There should always be a change in the preparation when the ASNase activity is less than the desired limit of 0.1IU/mL. For patients receiving multiple doses of E. coli ASNase and Erwinia ASNase, a desirable activity level of ≥0.1IU/mL is considered before each dose. In the case of PEG-ASNase, activity levels should be checked after 7 and 14 days and should be ≥0.1IU/mL.17 Silent inactivation can also happen, and its identification requires the real time measurement of either anti-ASNase antibodies or serum ASNase activity levels.13
Methods of analysis of hypersensitivity and inactivation processesCurrently, there are three main ways of analyzing the hypersensitivity and inactivation processes measurement of the ASNase activity, measurement of the serum asparagine levels and evaluation of the development of anti-ASNase antibodies.10,28,29 Of the existing methods of analysis, Anti-ASNase antibodies and asparagine measurements are not frequently used, since they are not directly useful in the clinical decision.10
Since the aim of ASNase therapy is asparagine depletion, the measurement of asparagine itself appears to be the most effective method of evaluating ASNase.17 However, accurate dosing of serum asparagine can be difficult due to the continuous hydrolysis of asparagine by the enzyme.29 For this reason, measuring asparagine levels is a difficult strategy.17 Assays measuring asparagine concentrations during asparaginase therapy have significant technical limitations. The measurement of asparagine in patients undergoing asparaginase therapy can be difficult due to the continued hydrolyzation of asparagine by the enzyme.14
In this context, the ASNase activity is often easier to measure and has been shown to strongly correlate with asparagine concentrations, besides being reproducible and reliable.17,28,29 The catalytic activity of ASNase is influenced by pH, molarity, and buffer additives.30 However, there are some disadvantages in measuring the activity of ASNASE as it does not represent the catalytic activity in human serum because the serum factors that can modulate the activity of different ASNASE preparations are probably masked by the dilution of the sample. The drug monitoring of ASNase activity does not describe the enzyme activity in vivo, but is equivalent to serum concentrations of the functional enzyme.30
On the other hand, there are some authors who suggest that the measurement of antibody levels could be used to predict which patients might benefit most from switching ASNase formulations. Willer et al. showed in a recent study that patients with high antibodies to native E. coli ASNase showed a significant reduction in ASNase activity during second-line treatment with PEG-ASNase, highlighting the cross reactivity between these two enzymes, while patients with moderate native E. coli antibody levels still showed ASNase activity >0.1IU/mL, when exposed to PEG-ASNase.26
The therapeutic drug monitoring may further optimize ASNase treatment, as greater levels of ASNase activity have been associated with improved outcomes.31 Through these techniques, practitioners can evaluate whether an individual patient needs to switch ASNase due to subclinical hypersensitivity or silent inactivation.16,32
For the reasons stated above, the method more frequently used to adapt treatment is ASNase activity measurement. In 2013, Vrooman et al. defined silent inactivation as two consecutive measurements of through ASNase activity <0.1IU/ml.32 More recently, Van der Sluis et al. stated that silent inactivation can be identified by the assessment of serum ASNase activity, preferably measured in 2 independent samples.17
Management in cases of hypersensitivity or inactivationAll patients who receive any ASNase preparation should be observed for at least 1h following the administration to monitor any adverse reaction. Due to the longer half-life of PEG-ASNase, hypersensitivity reactions may appear many hours after the administration of the drug.33,34
It is described that failure to receive the complete course of ASNase treatment due to hypersensitivity, other toxicities, or intolerable side effects, has been associated with inferior outcomes in ALL, compared with those who receive the majority of the intended dose of ASNase.31 In an international consensus, worldwide authorities agreed that when patients develop a clinical allergy, the preparation has to be switched, while if there is any doubt, the activity should be measured.
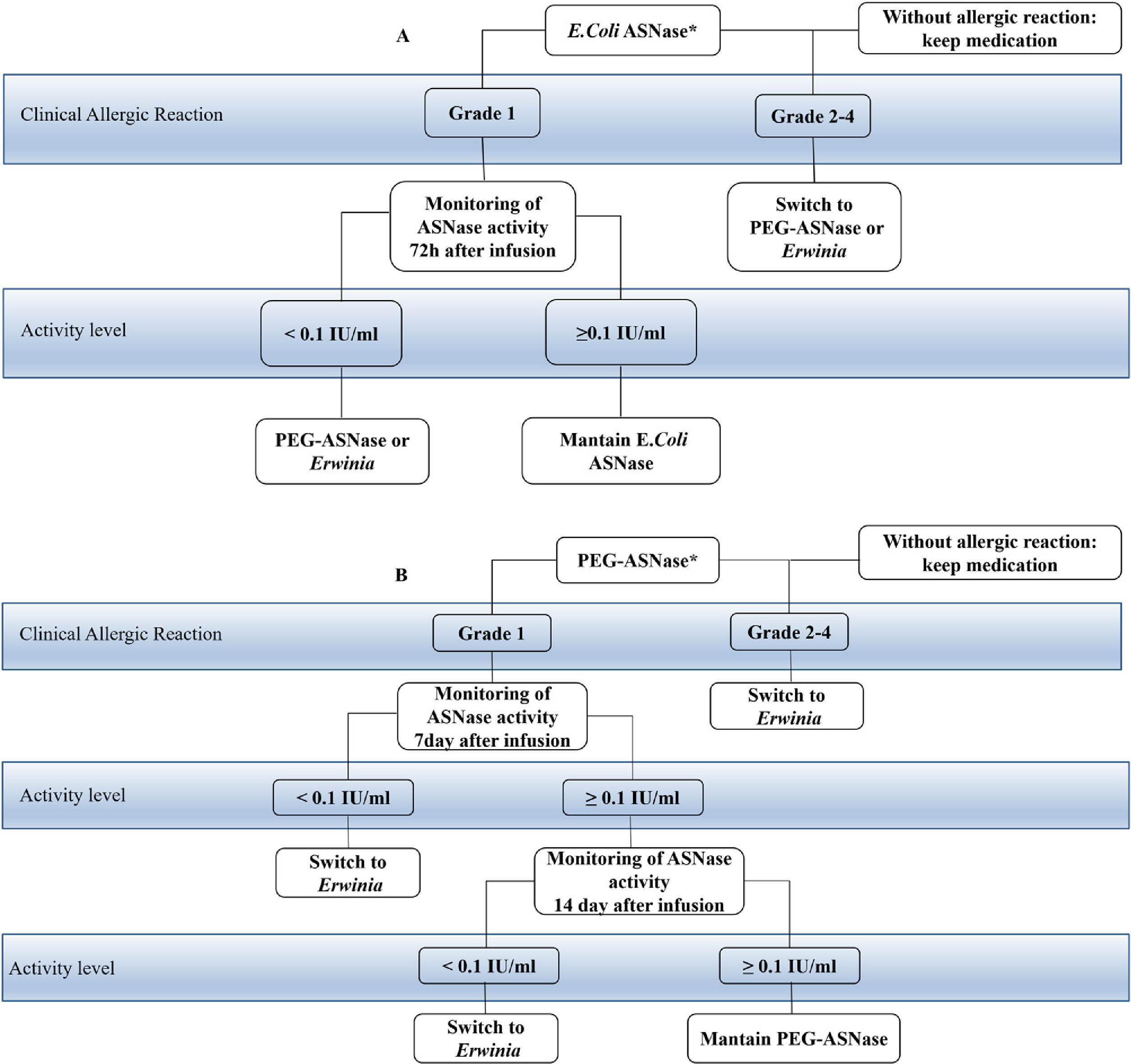
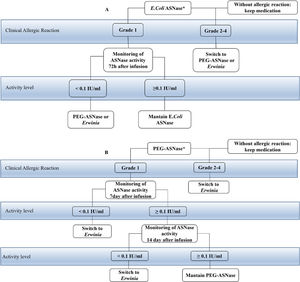
The consensus recommends that patients who develop grade 1 allergic reactions after intravenous administration monitor the activity of ASNase in real time to identify silent inactivation, if it occurs. In patients with grade 2–4 allergic reactions, according to the CTCAE criteria, after intravenous or intramuscular administration, the ASNase preparation change is indicated, without the need for activity tests. It is recommended that when the patient develops any questionable reaction with intramuscular administration, the activity of ASNase should be checked.17 They define that screening for silent inactivation should be considered for all patients undergoing asparaginase ALL therapy. Monitoring of activity in E. coli ASNase should be performed after the first dose and after each reintroduction and the PEG ASNase should be done within 7 days. If the level is detectable, but less than 0.1IU/mL, the activity should be checked again on day 14, as described in Fig. 1, that shows the change of treatment options when any hypersensitivity reaction occurs or when there is silent inactivation. Patients who develop some hypersensitivity reaction to the E. coli native preparation should have it replaced by PEG ASNase or Erwinia. The choice of a second-line agent depends on the protocol specifications and availability. Patients who initially received PEG-ASNase may only be switched to Erwinia.17
Taken according to the initial drug used. In patients who develop grade 2 to 4 allergic reactions, the preparation is switched without activity monitoring. In patients who present grade 1 reactions or questionable reactions, the monitoring of the activity that varies according to the initial drug used is indicated. (A) the starting drug is E. coli ASNase, monitoring should be done after the first dose and after each reintroduction, which usually takes 72h. If it has activity <0.1IU/ml, it is suggested to switch to another formulation, PEG-ASNase or Erwinia. But if it has activity ≥0.1IU/ml they maintain the infusions of E. Coli ASNase. (B) the starting drug is PEG-ASNase, monitoring is indicated at day 7 after infusion. If the patient exhibits activity <0.1IU/ml, swicth for Erwinia is suggested. If the activity is ≥0.1IU/ml, it is monitored again on day 14, if the patient has activity <0.1IU/ml, it is suggested to change to Erwinia and if it has activity ≥0.1IU/ml maintain the infusions of PEG-ASNase.
The development of anti-ASNase antibodies is most commonly observed with native E. coli ASNase.16 Clinical hypersensitivity to native E. coli ASNase has been reported in up to 75% of patients with ALL.20 It appears to be less prevalent with PEG-ASNase, with rates from 3 to 24%, as reported in clinical trials.30,31,35 Hypersensitivity reactions to PEG-ASNase are more common when patients have been previously exposed to native E. coli ASNase.20 In such cases, substitution is indicated for a different preparation which may prolong ASNase therapy and possibly improve patient outcomes.36 Neutralizing antibodies produced against the preparation of native E. coli have shown a cross-reaction with PEG-ASNase, but not with Erwinia ASNase.22 Successful ASNase substitutions require that there be no or little cross-reactivity with the offending preparations.13 For these reasons, Erwinase is generally used as a second- or third-line treatment to replace E. coli ASNase when severe allergic reactions occur because there is virtually no cross-reaction between the two products.18,33,37 These data lead to a strategy that allows the majority of patients to complete their prescribed treatment regimen even when they experienced hypersensitivity to native E. coli asparaginase or PEG-asparaginase.38
In a recent report on cognitive function tests (COG), 55 patients who developed hypersensitivity to PEG-ASNase were switched to Erwinia chrysanthemi. The goal of this study was to evaluate both 48- and 72h asparaginase activity levels to determine if the current dose resulted in sufficient asparaginase activity. Results showed that asparaginase Erwinia chrysanthemi was well tolerated and, importantly, maintained asparaginase activity >0.1IU/ml at both 48 and 72h post-injection in all patients tested.24In patients who switch to ASNase Erwinia chrysanthemi, outcomes were similar to patients who never develop clinical hypersensitivity.10 Rates of clinical hypersensitivity in patients receiving Erwinia ASNase have been reported to be 3–37% of patients.20,35
As already mentioned, it is important to notice that the use of pre-medication, such as steroids and antihistamines or decreasing the infusion rate, should be avoided, as these measures reduce allergic symptoms, but do not prevent inactivation of ASNase by antibodies.10,17,18
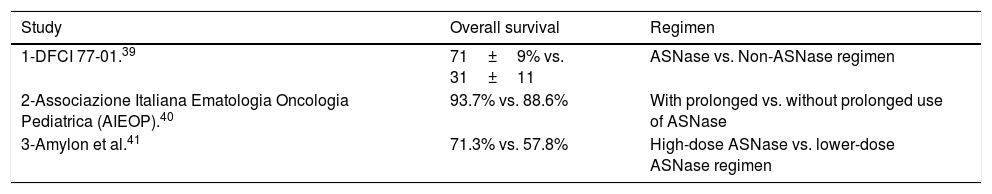
Clinical applicationSeveral studies showed the historical importance of the use of asparaginase in ALL treatment, as shown in Table 2. In the 70s, the Dana-Farber Cancer Institute (DFCI) 77-01 study compared patients treated with or without ASNase therapy, as part of a multiagent chemotherapy regimen. Patients in the arm that included native E. coli ASNase during intensification therapy showed greater overall survival rates in the 9.4-year follow-up, compared with patients who did not receive ASNase.39
Importance of the use of asparaginase comparing different therapeutic protocols.
| Study | Overall survival | Regimen |
|---|---|---|
| 1-DFCI 77-01.39 | 71±9% vs. 31±11 | ASNase vs. Non-ASNase regimen |
| 2-Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP).40 | 93.7% vs. 88.6% | With prolonged vs. without prolonged use of ASNase |
| 3-Amylon et al.41 | 71.3% vs. 57.8% | High-dose ASNase vs. lower-dose ASNase regimen |
The randomized study carried out by the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) determined the efficacy of a BFM-type chemotherapy regimen with or without the prolonged use of high-dose native E. coli asparaginase during continuation therapy. Children given asparaginase had a significantly increased 10-year disease-free survival (87.5% vs. 78.7%).40.
Data of Amylon et al. showed that high-dose native E. coli asparaginase during consolidation significantly improved complete continuous remission in pediatric patients with ALL and lymphoblastic lymphoma, compared with patients treated with lower-dose asparaginase regimen.41 Through these previously described studies, the importance and benefits of asparaginase treatment became clear.32
There are many advantages in performing the monitoring of ASNase activity.17 Among them, we may describe the possibility of optimizing dosing schedules of different ASNase preparations, identifying patients with pseudo-allergic reactions who can continue their therapy and identifying patients with silent inactivation.42 This strategy allows for an improvement in care, with which patients will be able to adapt their treatment according to international protocols to improve results.18,26
Some studies showed the importance of therapeutic monitoring, by correlating the levels of antiasparaginase antibodies with their activity or only measuring activity. The study conducted by Avramis et al.30 determined the correlation between antibody levels and the proportion of samples with asparaginase activity for asparagine depletion (≥0.1IU/mL) at each stage of therapy in patients receiving either native E. coli or PEG-ASNase. The majority of the children (89–93% depending on the stage of therapy) with low antibody levels had asparaginase activity ≥0.1IU/mL. Only 50–64% of children with high antibody levels had asparaginase activity ≥0.1IU/mL. In the study conducted by Vrooman et al.,32 patients treated with native E. coli were randomized into two groups, one with individualized dosing, and the other with fixed dosing. Patients in the individualized group with ASNase activity levels <0.1IU/mL despite dose adjustment were considered to have silent inactivation and were switched to another ASNase preparation. In the group with the fixed dosage, only the preparation was changed when clinical hypersensitivity occurred. Patients in the first group had significantly greater event-free survival at five years than patients in the second group (90% vs. 82%, respectively). This improvement in survival was probably due to the detection of silent inactivation in 10% of the patients in the first group and the change to another ASNase.
A study published by Schrey et al.43 reported results of therapeutic monitoring of asparaginase activity in 127 patients. First-line therapy included native E. coli, which resulted in ASNase activity <0.1IU/mL in 7% of patients in induction and 29% in reinduction. Second-line treatment for patients with clinical allergy or silent inactivation was PEG-ASNase, which resulted in asparaginase activity <0.1IU/mL in 17% of patients of these patients.
In our recent study on a sample of Brazilian patients, has we were able to clearly show the importance of measuring drug activity in patients with ALL in our reality. We analysed 262 serum samples taken 24h and 48h after infusions of an asparaginase preparation. We were able to detect a large group of patients whose asparaginase activity was lower than 0.1IU/mL This data highlighted the importance of monitoring asparaginase activity in midle income countries. This kind of analysiscan help policy makers to establish the appropriate strategies to provide access to efficient treatment for all patients.44
ConclusionsASNase has historically been a critical component of multi-agent chemotherapy for the treatment of ALL. Intensified ASNase use is associated with significant improvements in outcomes for patients with ALL.
The possibility of switching ASNase formulations in ALL patients with clinical hypersensitivity allows for the completion of the scheduled ASNase treatment and has been shown to significantly improve survival. The monitoring of ASNase activity in patients can be used to identify ASNase levels considered inadequate for asparagine depletion, as well as to identify silent inactivation in patients who may not display clinical hypersensitivity. The ability to individualize the ASNase therapy in patients, adjusting the dose, or switching patients with silent inactivation to an alternate ASNase preparation, may help improve the outcomes in those patients.
There are few studies in our reality that evaluate the asparaginase activity in the various formulations available. The identification of silent inactivation is probably an effective and easily available strategy to improve outcome of children and adolescents with ALL.
Conflicts of interestThe authors declare no conflicts of interest.