The Acute Leukemia-European Society for Blood and Marrow Transplantation (AL-EBMT) risk score was recently developed and validated by Shouval et al.
ObjectiveTo assess the ability of this score in predicting the 2-year overall survival (OS-2), leukemia-free survival (LFS-2) and transplant-related mortality (TRM) in acute leukemia (AL) adult patients undergoing a first allogeneic hematopoietic stem cell transplant (HSCT) at a transplant center in Brazil.
MethodsIn this prospective, cohort study, we used the formula published by Shouval et al. to calculate the AL-EBMT score and stratify patients into three risk categories.
ResultsA total of 79 patients transplanted between 2008 and 2018 were analyzed. The median age was 38 years. Acute myeloid leukemia was the most common diagnosis (68%). Almost a quarter of the cases were at an advanced stage. All hematopoietic stem cell transplantations (HSCTs) were human leukocyte antigen-matched (HLA-matched) and the majority used familial donors (77%). Myeloablative conditioning was used in 92% of the cases. Stratification according to the AL-EBMT score into low-, intermediate- and high-risk groups yielded the following results: 40%, 12% and 47% of the cases, respectively. The high scoring group was associated with a hazard ratio of 2.1 (p = 0.007), 2.1 (p = 0.009) and 2.47 (p = 0.01) for the 2-year OS, LFS and TRM, respectively.
ConclusionThis study supports the ability of the AL-EBMT score to reasonably predict the 2-year post-transplant OS, LFS and TRM and to discriminate between risk categories in adult patients with AL, thus confirming its usefulness in clinical decision-making in this setting. Larger, multicenter studies may further help confirm these findings.
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment for acute leukemia (AL). Despite the reduction in transplant-related risk during the last few years, it is still associated with significant morbidity and mortality, which raises the question of who, how and when to transplant.
Transplant-related mortality (TRM) is mainly due to graft-versus-host disease (GVHD), infection and organ toxicity. Several factors are known to affect TRM and survival among HSCT patients, such as those related to the patient (e.g., age and comorbidities), baseline disease (e.g., disease stage at the time of transplant and cytogenetic features), donor (i.e., human leukocyte antigen (HLA) match between donor and recipient) and the procedure itself (e.g., type of conditioning regimen).1–3 Since TRM differs considerably among HSCT recipients, a prognostic scoring system which can potentially guide patient counseling and clinical decision-making is strongly advised before proceeding to transplant.
Different scoring systems have already been devised for predicting HSCT outcomes, including the European Society for Blood and Marrow Transplantation (EBMT) risk score and the Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI).2,4 Although such scores do help in the clinical decision-making process, their predictive ability varies widely across different settings.2,4-6
In 2015, Shouval et al.7 developed and validated the Acute Leukemia – EBMT (AL-EBMT) score for the prediction of mortality following HSCT. This prognostic model comprised 10 variables related to the patient, donor, type of transplant, type of leukemia, disease stage at the time of transplant, year of transplantand annual HSCT center activity. In this study, the AL-EBMT score provided an individualized estimate of the probability of mortality within the first 100 days post-transplant in AL patients. It was also shown to be capable of predicting overall survival (OS), event-free survival (EFS-2) and TRM at 2 years after HSCT.7,8
Therefore, we proposed to evaluate the ability of the AL-EBMT risk score in predicting the 2-year OS, EFS and TRM in AL patients undergoing an HSCT at a reference transplant center in Brazil.
MethodsStudy designThis was a single-center cohort study, which was undertaken at the HSCT unit of Hospital das Clínicas (HC), Federal University of Minas Gerais (UFMG), Belo Horizonte, Brazil. This is a reference center in this country, with an estimated annual HSCT activity of approximately 30 allogeneic transplants, twothirds of which are performed in adults. The inclusion period comprised all consecutive allogeneic adult transplants for AL performed from March 1, 2008 until December 31, 2018. The included patients were followedup on prospectively and we applied the AL-EBMT formula published by Shouval et al.7
This study was approved by the Research Ethics Committee of UFMG (protocol number: CAAE – 2172313.8.0000.5149) and all study participants signed an informed consent form, in accordance with the Declaration of Helsinki.
Study participants and the AL-EBMT scoreThe inclusion criteria were defined as patients aged 18 years or over, who had a diagnosis of AL and were to undertake a first HSCT. Hematopoietic stem cell sources were bone marrow (BM) and peripheral blood stem cells (PBSCs) from related and unrelated donors. Patients receiving umbilical cord blood as the stem cell source and haploidentical transplantation were excluded from this analysis.
We used the freely available formula published by Shouval et al. (http://bioinfo.lnx.biu.ac.il/~bondi/web1.html)7 to calculate the AL-EBMT score. This formula estimates the 100-day post-transplant probability of mortality based on the following factors at the time of transplant: age of the recipient, type of acute leukemia (acute myeloid leukemia(AML) vs. acute lymphoblastic leukemia ALL)), disease stage (first complete remission(CR1); second complete remission(CR2); all other disease stages; or unknown), Karnofsky Performance Status (KPS) (< 80% vs. ≥ 80%, or unknown), donor-recipient cytomegalovirus (CMV) serostatus combination (both negative; both positive; one negative and the other positive; or unknown), conditioning regimen (myeloablative conditioning (MAC) vs. reduced intensity conditioning (RIC), or unknown), type of donor (Human Leukocyte Antigen,HLA-matched related, vs. matched unrelated donor, or unknown), interval between diagnosis and transplant (less than 142 days or not, or unknown)and average annual number of transplants during the last three years of recruitment at the HSCT center (< 20 or ≥ 21, or unknown). According to the AL-EBMT score, participants were stratified into three groups: low-risk (< 8.5), intermediate (8.6–10.0) and high- risk (> 10.0) categories.
For the definition of MAC regimens, we used the criteria proposed by the National Marrow Donor Program (NMDP) and the Center for International Blood and Marrow Transplant Research (CIBMTR)9,10, in which a total busulfan dose of ≥ 9.0 mg/kg (oral formulation) or ≥ 7.2 mg/kg (intravenous (IV) formulation) and/or a total IV melphalan dose of ≥ 140 mg/m2 of body surface area and/or TBI ≥ 5 Gy (single dose) or ≥ 8 Gy (fractionated) were considered myeloablative. Conditioning regimens not fulfilling any of these criteria were considered RIC regimens.
An HLA-identical transplant was defined as an 8/8 HLA-match (for HLA-A and HLA-B at antigenic/allele level and HLA-DR and HLA-DQ at allele level) between donor and recipient for related donorsand a 10/10 HLA-match (for HLA-A, HLA-B and HLA-C at antigenic/allele level and HLA-DR and HLA-DQ at allele level) for unrelated donors.
OutcomesAll the results were measured considering the time of transplant as baseline. The TRM-2 was defined as death without relapse or progression at two years post-transplant. The EFS-2 comprised the interval between the time of transplant and the occurrence of any event (death or relapse) during the first two years of transplant. The OS-2 was considered as the probability of being alive at two years post-HSCT.
Statistical analysisDescriptive statistics included frequency (N) and proportions for categorical variables, whereas medians and interquartile ranges (IQRs) were used for continuous variables. The Kaplan-Meier method was used for estimating LFS-2 and OS-2and the log-rank test was used for the comparison between groups. Data were censored at the time of death or of last follow-up according to medical records. The Gray's method was used for the analysis of the incidence of competing events. In the analysis of the cumulative incidence of the TRM, relapse was considered as a competing event. A confidence level of 95% was used for the hazard risk (HR) estimates (95% confidence interval (95% CI)). The Cox's proportional hazards regression model was used for the hazard risk estimates of the EFS-2 and OS-2 between the AL-EBMT groups, whereas the Fine and Gray method was used for the HR estimates of competing events (TRM-2) between the AL-EBMT groups. A significance level of 0.05 was used. All data analyses were performed using the Easy R software package.
ResultsSeventy-nine patients were included, 44 (55.7%) of whom were male and the median age was 38 years (IQR: 27–47). AML was the most common diagnosis, accounting for 54 (68.4%) cases. Approximately a quarter (27.8%) of the AL cases were at an advanced stage (i.e., > CR2) at the time of transplant. All allogeneic transplants were HLA-matched, wherein familial donors accounted for the majority (77.2%) of cases. Stratification according to the AL-EBMT score into low-, intermediate- and high-risk groups yielded 32 (40.5%), 10 (12.7%) and 37 (46.8%) patients in each of the groups, respectively. These and other patient characteristics are shown in Table 1.
Patient baseline characteristics (N = 79).
| Variable | Value | |
|---|---|---|
| Age at transplant, median in years (IQR) | 38 | (27–47) |
| Interval between diagnosis and transplant, median in days (IQR) | 696 | (209–753) |
| Diagnosis n (%) | ||
| AML | 54 | (68.4) |
| ALL | 25 | (31.6) |
| Disease stage, n (%) | ||
| CR1 | 37 | (46.9) |
| CR2 | 20 | (25.3) |
| Advanced* | 22 | (27.8) |
| Recipient Sex, n (%) | ||
| Male | 44 | (55.7) |
| Female | 35 | (44.3) |
| Donor Type, n (%) | ||
| Related | 61 | (77.2) |
| Unrelated | 18 | (22.8) |
| Conditioning regimen, n (%) | ||
| MAC | 73 | (92.4) |
| RIC | 6 | (7.6) |
| Stem cell source, n (%) | ||
| Bone marrow | 17 | (21.5) |
| Peripheral blood | 62 | (78.5) |
| CMV serostatus (recipient), n (%) | ||
| Positive | 70 | (88.6) |
| Negative | 9 | (11.4) |
| CMV serostatus (donor), n (%) | ||
| Positive | 63 | (79.7) |
| Negative | 12 | (15.2) |
| Unknown | 4 | (5.1) |
| Donor-recipient serostatus combination, n (%) | ||
| Both negative | 3 | (3.8) |
| Both positive | 57 | (72.1) |
| Positive/Negative | 6 | (7.6) |
| Negative/Positive | 9 | (11.4) |
| Unknown/Positive | 4 | (5.1) |
| Karnofsky performance status, n (%) | ||
| ≥ 80% | 76 | (96.2) |
| < 80% | 3 | (3.8) |
| AL-EBMT score, n (%) | ||
| Low | 32 | (40.5) |
| Intermediate | 10 | (12.7) |
| High | 37 | (46.8) |
AL-EBMT score: acute leukemia – European society for blood and marrow transplantation score; CMV: cytomegalovirus; IQR: interquartile range; ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; MAC: myeloablative conditioning (regimen); RIC: reduced intensity conditioning (regimen); CR1: first complete remission; CR2: second complete remission.
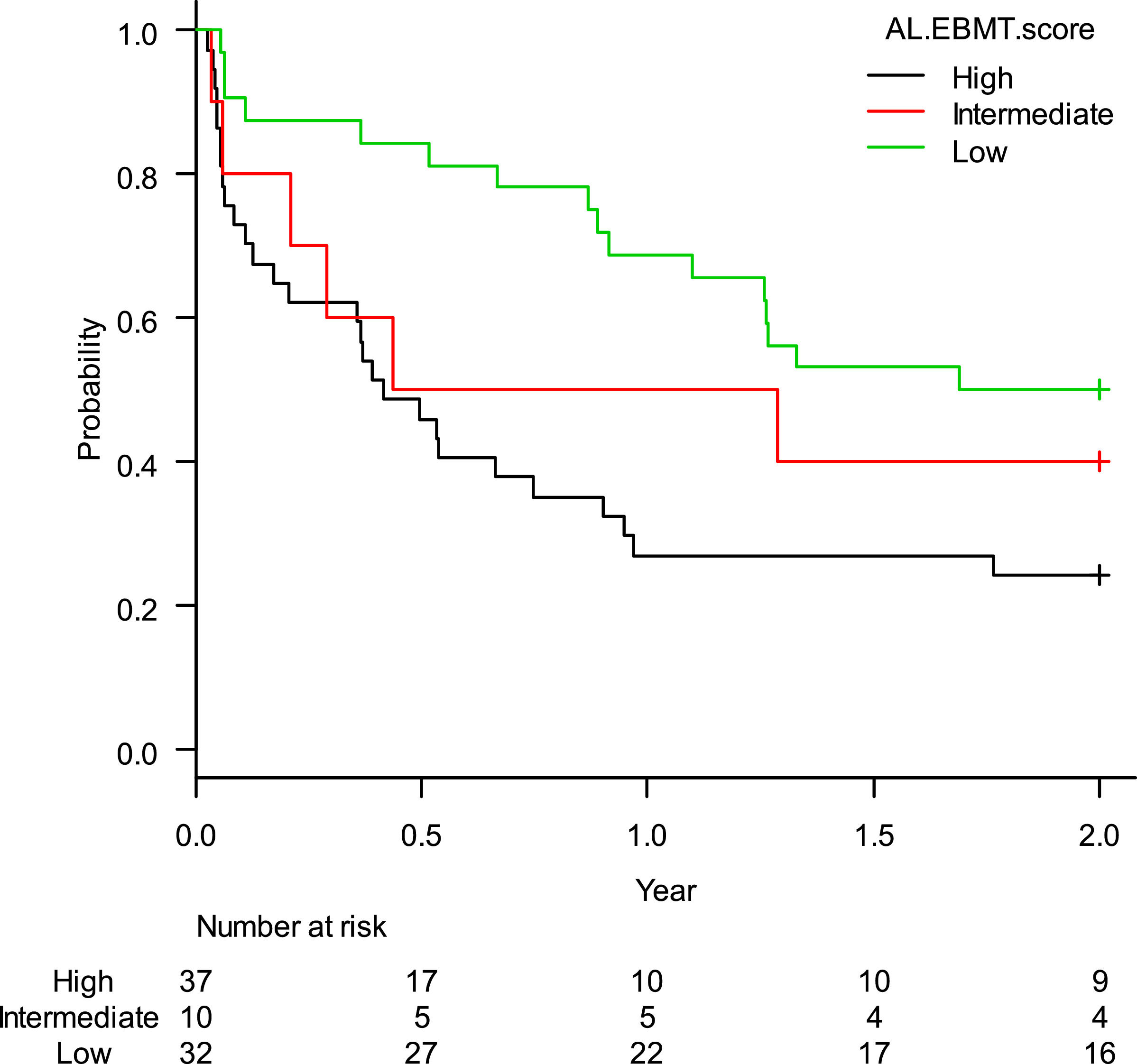
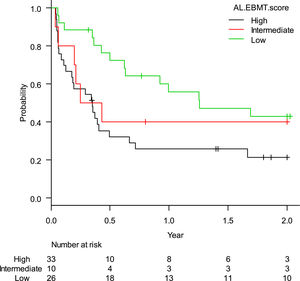
The estimated rate of OS-2 was 36.7% (95%CI: 26.2–47.2%). When stratified according to the AL-EBMT score, low-, intermediate- and high-risk groups had an estimated OS-2 of 50.0% (95%CI: 31.9–65.7%), 40.0% (95%CI: 12.3–67.0%), and 24.3% (95%CI: 12.1–38.8%), respectively; p = 0.01 (Figure 1). Comparison of low versus intermediate/high and of high versus low/intermediate AL-EBMT scores showed an OS-2 HR of 0.42 (95%CI: 0.22–0.78; p = 0.006) and 2.14 (95%CI: 1.22–3.76; p = 0.007), respectively (Table 2).
Hazard ratios according to each AL-EBMT risk group - HC-UFMG cohort (2008–2018).
AL-EBMT: Acute leukemia- European society for blood and marrow transplantation score; HR: hazard ratio; HC-UFMG: hospital das clínicas – federal university of minas gerais; Ref: reference; OS-2: overall survival at 2 years (post-transplant); LFS-2: leukemia-free survival at 2 years (post-transplant); TRM-2: transplant-related mortality at 2 years (post-transplant).
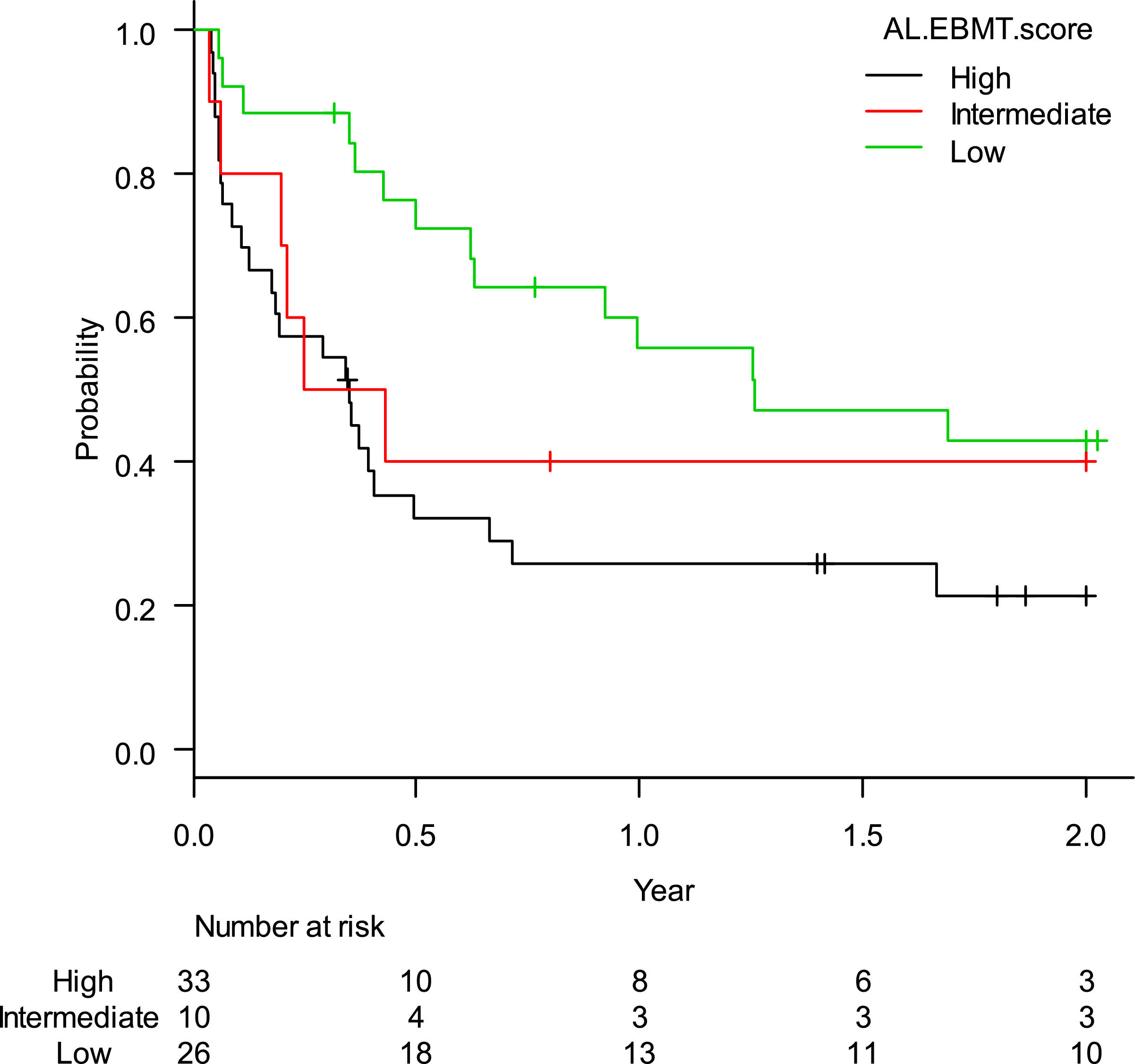
The estimated rate of EFS-2 was 32.3% (95%CI: 21.3–43.8%). When stratified according to the AL-EBMT score, low-, intermediate- and high-risk groups had an estimated LFS-2 of 42.9% (95%CI: 23.2–61,2%), 40.0% (95%CI: 12.3–67.0%), and 21.5% (95%CI: 9.0–37.5%), respectively; p = 0.04 (Figure 2). Comparison of low versus intermediate/high and of high versus low/intermediate AL-EBMT scores showed an EFS-2 HR of 0.42 (95%CI: 0.22–0.78; p = 0.006) and 2.10 (95%CI: 1.20 - 3.69; p = 0.009), respectively (Table 2).
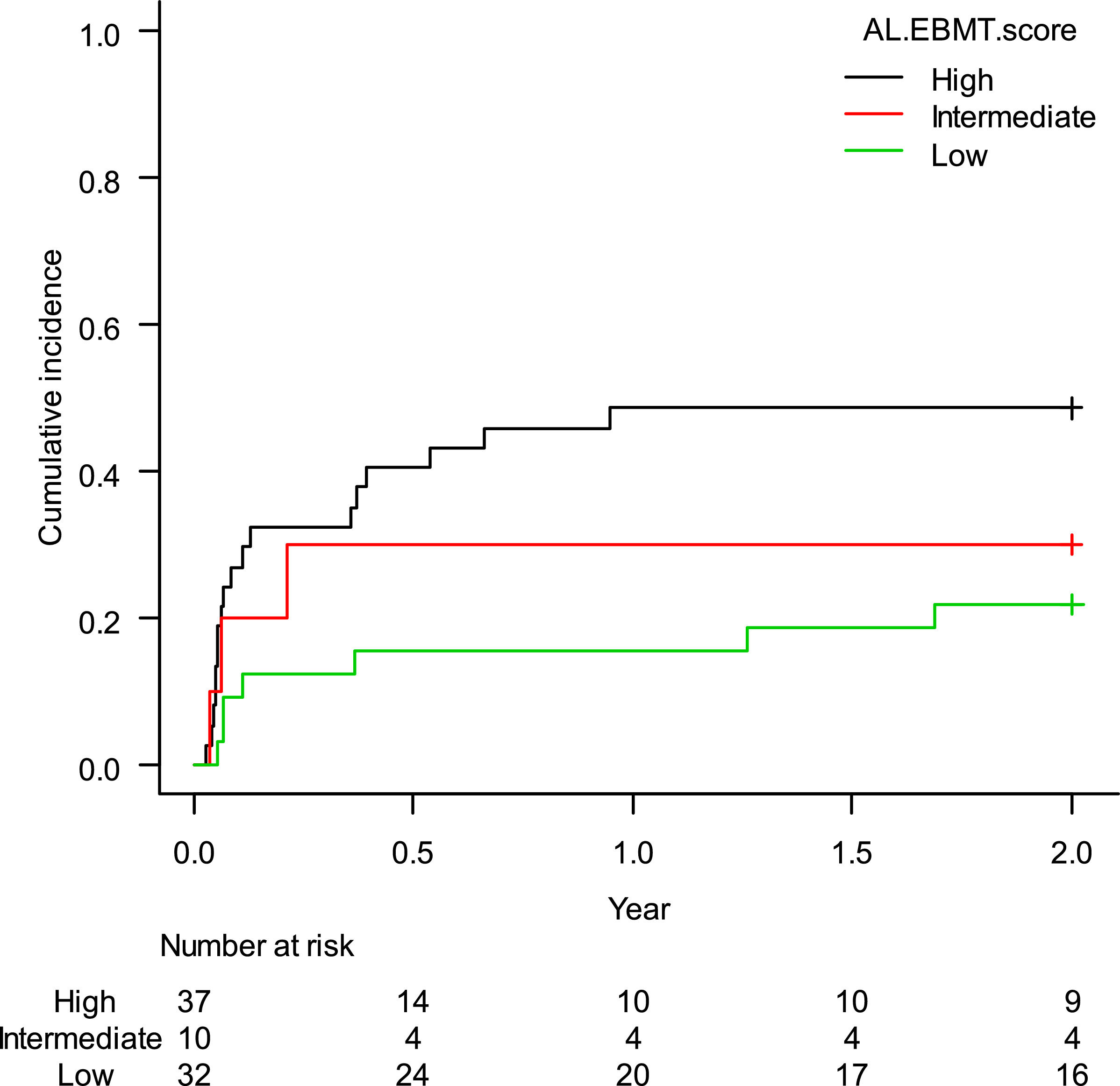
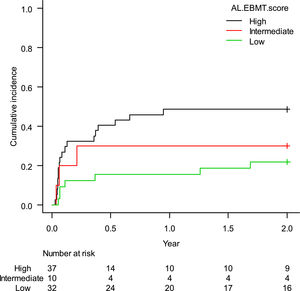
The estimated cumulative incidence of TRM-2 was 35.4% (95%CI: 25.5–46.0%). When stratified according to the AL-EBMT score, low-, intermediate- and high-risk groups had an estimated TRM-2 of 21.9% (95%CI: 9.4–37.6%), 30.0% (95%CI: 6.2–59.4%), and 48.6% (95%CI: 31.5–63.8%), respectively; p = 0.05 (Figure 3). Comparison of low versus intermediate/high and of high versus low/intermediate AL-EBMT scores showed a TRM-2 HR of 0.39 (95%CI: 0.17–0.91; p = 0.03) and of 2.47 (95%CI: 1.15–5.28; p = 0.01), respectively (Table 2).
DiscussionIn this single-center, prospective cohort study, the AL-EBMT score proposed by Shouval et al.7 was shown to be seemingly applicable as a prognostic model for OS, LFS and TRM at two years post-HSCT in patients with AL, based on specific, assessable risk factors at the time of transplantation.
In their original, retrospective analysis of EBMT registry data on 28,236 adult patients who had been submitted to a first allogeneic HSCT for AL, Shouval et al.7 showed that the AL-EBMT score was able to provide a reasonable estimate of OS, LFS, and TRM in such patients. As in our study, most patients had AML (68% vs. 70%, respectively), with a similar proportion of disease at an advanced stage at the time of transplantation (28% vs. 21%) and of use of PBSC (78.5% vs. 78.3%, respectively). However, the study by Shouval et al.7 showed a greater proportion of unrelated donors (46.1% vs. 22.8% in ours) and less CMV seropositivity in the recipient (64.5% vs. 88.6%). Moreover, in their study, a more homogeneous distribution of the study population among the three AL-EBMT strata was observed; in the present study, a clear polarization between low-score (33% vs. 40.5% in our study) and high-risk (34.3% vs. 46.8%) categories was noted. This may at least partly explain the statistically significant results regarding the OS, LFS and TRM observed between these two categories in our study, as opposed to the absence of a significant discrimination between these two strata and the intermediate-risk category.
In a second, retrospective, multicenter, the Gruppo Italiano Trapianti di Midollo Osseo (GITMO) cohort study aiming to validate the AL‐EBMT score for the prediction of mortality following allogeneic HSCT by stratifying 1848 patients according to the previously mentioned risk categories,8 this prognostic model was shown to provide a reasonable estimate of the OS, LFS, and TRM both in the short-term (100 days post-HSCT) and at 2 years post-transplant. Compared to our study, that of Shouval et al. showed a similar predominance of patients with AML (68.4% and 68.1%, respectively) and CMV seropositivity (88.6% and 81.4%), but a higher proportion of AML in CR1 (46.9% vs. 60.4%) and of unrelated donor transplants (22.8% vs. 49.6%); in contrast, lesser use of PBSC (69.9% vs. 78.5%) was noted, when compared to that in the current study (Table 3). As in the EBMT study, about a third (32%) of the patients in the AL-EBMT cohort were stratified as high-risk, as compared to almost half (46.8%) the patients in our cohort. As mentioned before, given the low representativeness of our study sample with respect to the intermediate-risk stratum (only 14.5% of the patients), this may account for the fact that significant discrimination was only observed between the lower and higher risk categories in our study.
Comparison between the baseline characteristics of the Teixeira et al. and Shouval et al.8 study populations.
| Variable | Teixeira et al. (n = 79) | Shouval et al.8 (n = 1848) | ||
|---|---|---|---|---|
| Value | Value | |||
| Age at transplant, median in year (IQR) | 38 | (27–47) | 46 | (35–55) |
| Interval between diagnosis and transplant, median in days (IQR) | 696 | (209–753) | 236 | (166–415) |
| Diagnosis, n (%) | ||||
| AML | 54 | (68.4) | 1258 | (68.1) |
| ALL | 25 | (31.6) | 590 | (31.9) |
| Disease stage, n (%) | ||||
| CR1 | 37 | (46.9) | 966 | (60.4) |
| CR2 | 20 | (25.3) | 334 | (20.9) |
| Advanced* | 22 | (27.8) | 300 | (18.8) |
| Patient sex, n (%) | ||||
| Male | 44 | (55.4) | 1060 | (57.6) |
| Female | 35 | (44.3) | 781 | (42.4) |
| Donor Type, n (%) | ||||
| Related | 61 | (77.2) | 931 | (50.4) |
| Unrelated | 18 | (22.8) | 917 | (49.6) |
| Conditioning regimen, n (%) | ||||
| MAC | 73 | (92.4) | 1441 | (81.3) |
| RIC | 6 | (7.6) | 331 | (18.7) |
| Stem cell source, n (%) | ||||
| Bone marrow | 17 | (21.5) | 554 | (30.1) |
| Peripheral blood | 62 | (78.5) | 1285 | (69.9) |
| Karnofsky performance status, n (%) | ||||
| ≥ 80% | 76 | (96.2) | 1440 | (95.6) |
| < 80% | 3 | (3.8) | 65 | (4.4) |
| Patient CMV serostatus, n (%) | ||||
| Positive | 70 | (88.6) | 1265 | (81.4) |
| Donor- recipient CMV serostatus combination | ||||
| Both negative | 3 | (3.8) | 159 | (10.7) |
| Both positive | 57 | (72.1) | 815 | (34.5) |
| One negative/unknown and other positive | 19 | (24.1) | 514 | (34.5) |
CMV: cytomegalovirus; IQR: interquartile range; ALL: acute lymphoblastic leukemia; AML: acute myeloid leukemia; MAC: myeloablative conditioning (regimen); RIC: reduced intensity conditioning (regimen); CR1: first complete remission; CR2: second complete remission.
Compared with other prognostic scores used in HSCT, Shouval et al.8 demonstrated that the AL-EBMT and the Hematopoietic Cell Transplant- Comorbidity Index (HCT-CI) were independent predictors of the OS, LFS and non-relapse mortality (NRM). Of note, in their study, the AL-EBMT index was shown to provide a better estimate of the OS and LFS, while the HCT-CI seemed more effective in stratifying NRM between each score. Therefore, we believe these prognostic models may serve as a complement to another, given their additive properties. By providing a weighted account of a myriad of clinically relevant risk factors comprised within the HSCT scenario, including baseline-disease, transplant, and patient-related characteristics, such scoring systems, once used together, may better refine the clinical decision-making process prior to HSCT in adult patients with AL.
Our study has some important limitations. This was not a validation study, but a preliminary study aimed at evaluating the feasibility and applicability of the AL-EBMT score in adult patients with AL in a developing country. Its unicentric nature and relatively small sample size hamper any definitive conclusions regarding the robustness and generalizability of our results. In addition, the unbalanced baseline characteristics observed in our study may have contributed to the polarization noted between low- and high-risk AL-EBMT groups (the vast majority of the patients had AML as the underlying disease, PBSCs as the stem cell source, MAC as the preparative regimen, CMV seropositivity among donor-recipient pairs, high overall performance status and a predominance of related donors).
On the positive side, this is one of the first studies using prospective data to analyze the applicability of the AL-EBMT score in an underserved country, such as Brazil. We were able to demonstrate the feasibility and applicability of this score in this population, with results that may be potentially reproducible across other transplant centers.
ConclusionThe AL-EBMT score is a simple and freely available tool comprised of readily assessable variables, which makes it an important and useful clinical tool for the risk assessment of patients with AL referred to HSCT. Larger, multicenter studies combining other risk indices, such as the HCT-CI, and other transplant scenarios (e.g., haploidentical transplants) are strongly encouraged to better evaluate its predictive role and usefulness in clinical decision-making in the HSCT setting. The ease in its use and apparent reliability may favor its widespread applicability across centers worldwide.