γδ T-cell neoplasms are rare, including a variety of clinical pathological entities, and characterized by the expression of T-cell antigen receptors (TCRs), distributed in four types: T-cell acute lymphoblastic leukemia/lymphoma (T-ALL), hepatosplenic T-cell lymphoma (HSTCL), skin and mucosal T-cell lymphoma, and T-cells large granular lymphocytic leukemia (T-LGLL).1
The 2016 World Health Organization (WHO) classification of lymphoid neoplasms recognizes only two distinct entities of γδ T-cell: hepatosplenic γδ T-cell lymphoma and the γδ T-cell lymphoma. These neoplasms belong to a group of biologically distinct diseases, and they are usually clinically aggressive, with unfavorable prognosis. The γδ T-ALL represents 9 to 12% of T-ALL, including children and adults. Case reports of patients with T-ALL expressing the γδ TCR suggest that these patients also have a worse prognosis.2γδ T-ALL is a rare variant of T-ALL and compared to αβ T-ALL patients, usually present with lower hemoglobin concentrations in children, more frequent splenomegaly and higher WBC in adults, and higher percentages of the CD45RA−/CD45RO+ phenotype in all ages.3
We aimed to describe a case of γδ T-ALL after hematopoietic stem cell transplantation (HSCT) for AML due to the rarity of this diagnosis in adult patients, the difficulty of classification and the peculiarity of the post-transplant onset.
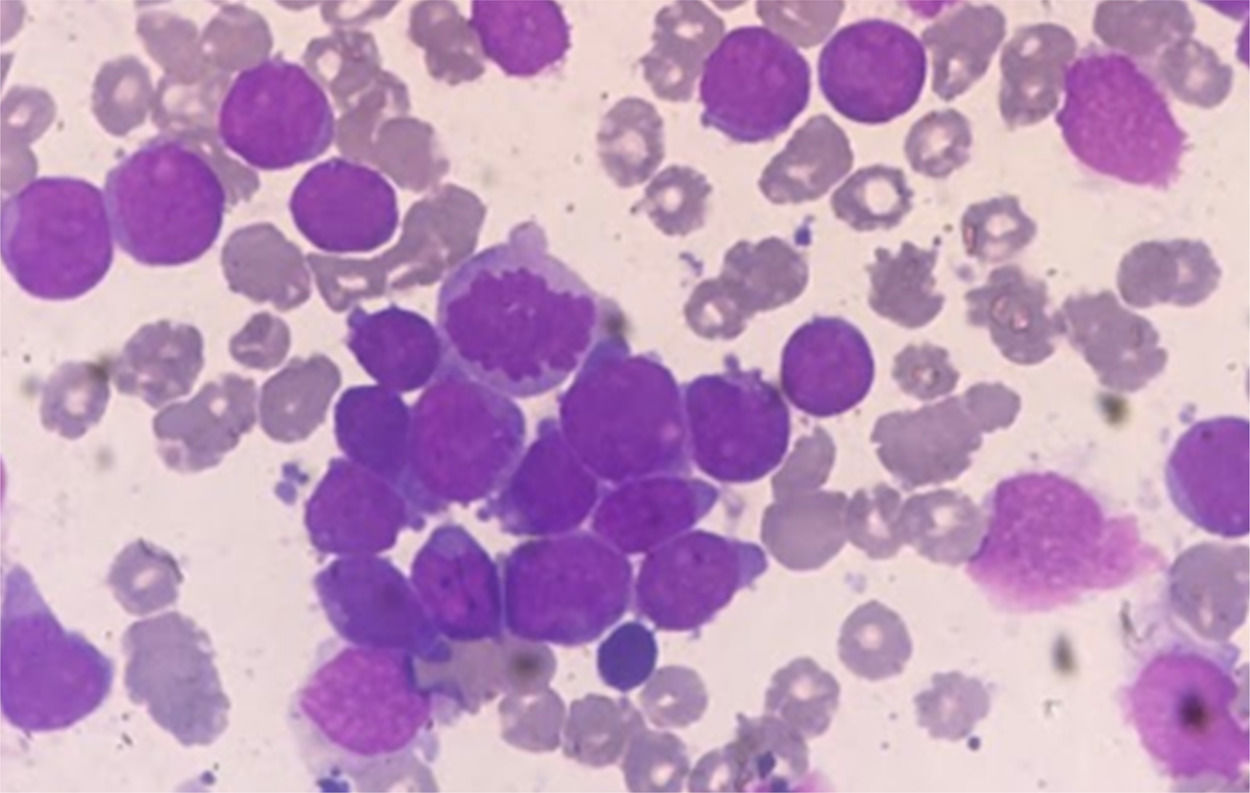
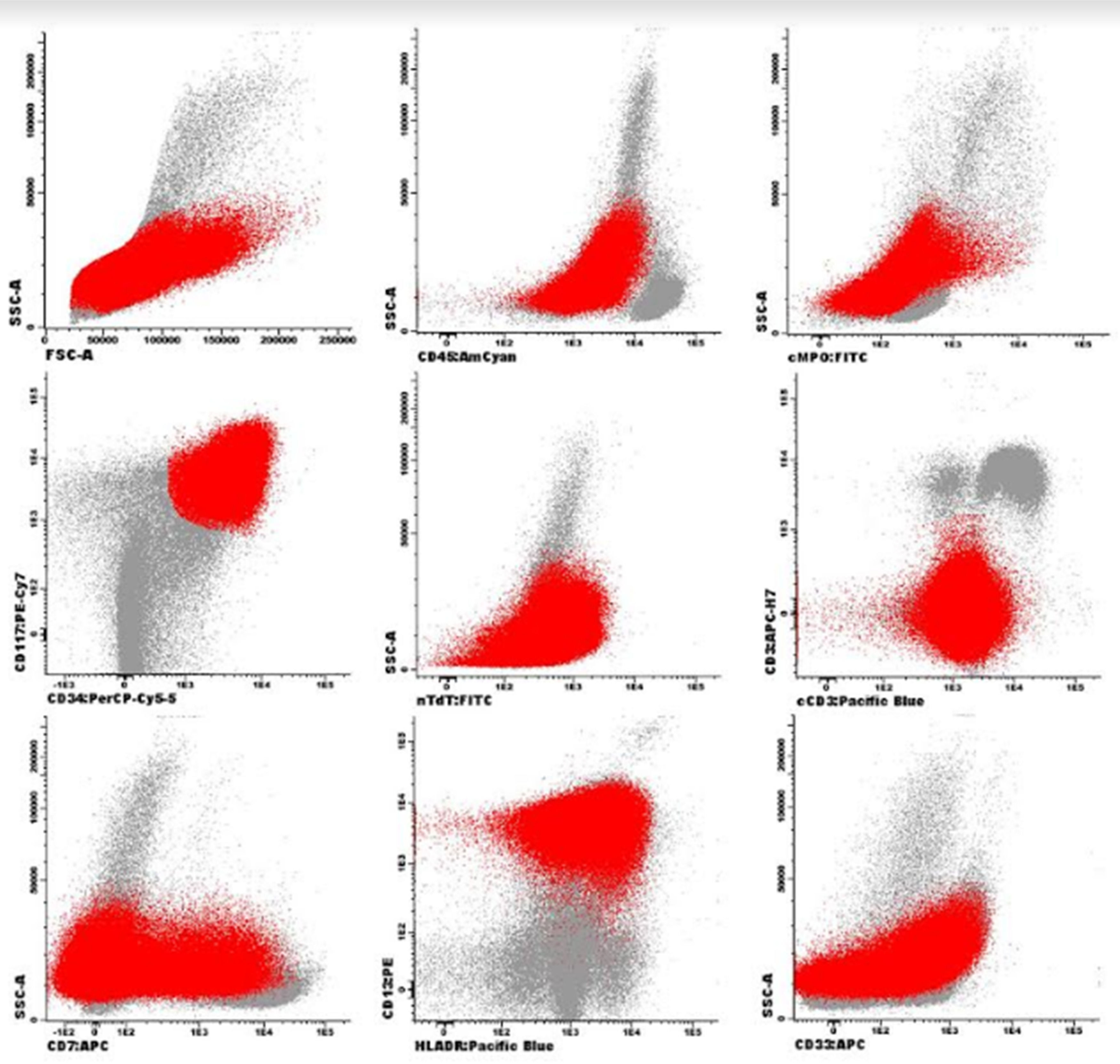
Case presentationFemale patient, 29-year-old, diagnosed with Acute Myeloid Leukemia (AML), in October 2017. Bone marrow aspirate showed infiltration by immature cells (Figures 1 and 3). Immunophenotyping demonstrated 74.0% of immature cells (CD34+, CD117+, HLADR+, CD33+ heterogeneous, CD13+strong, CD7+partial).
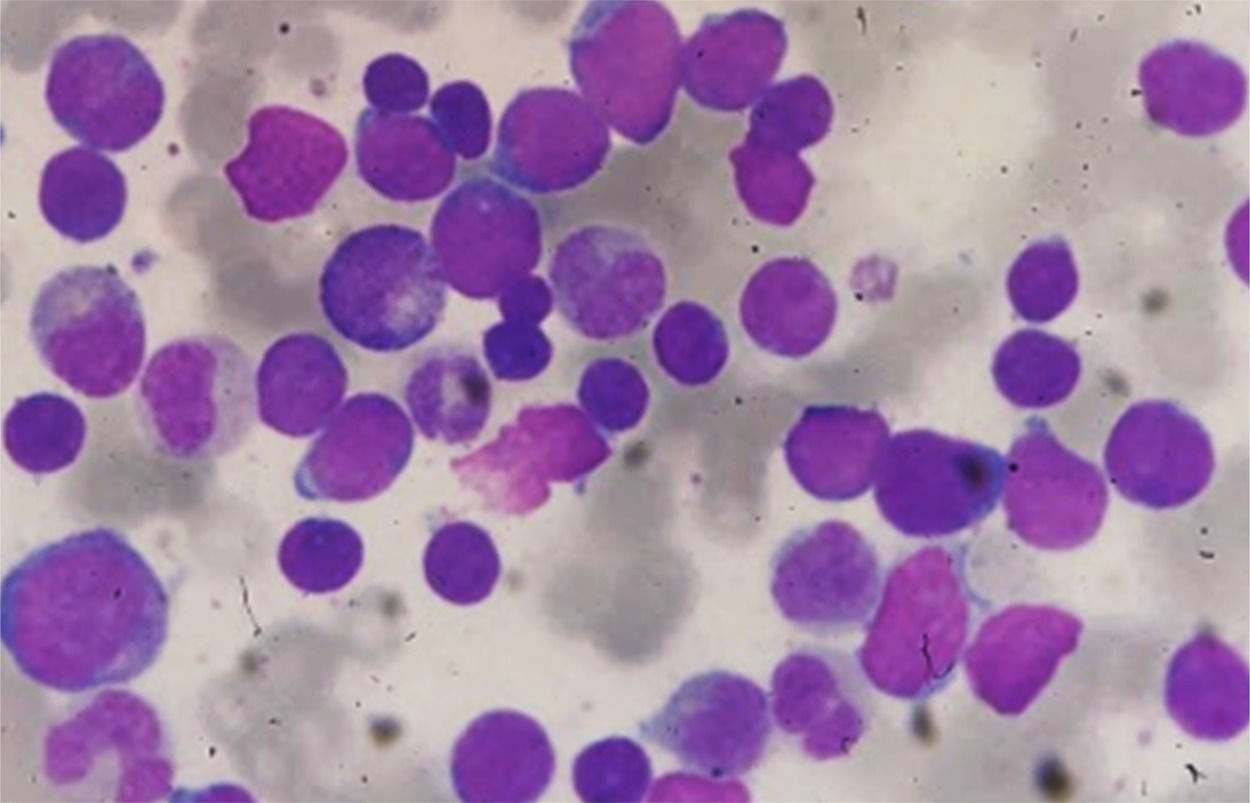
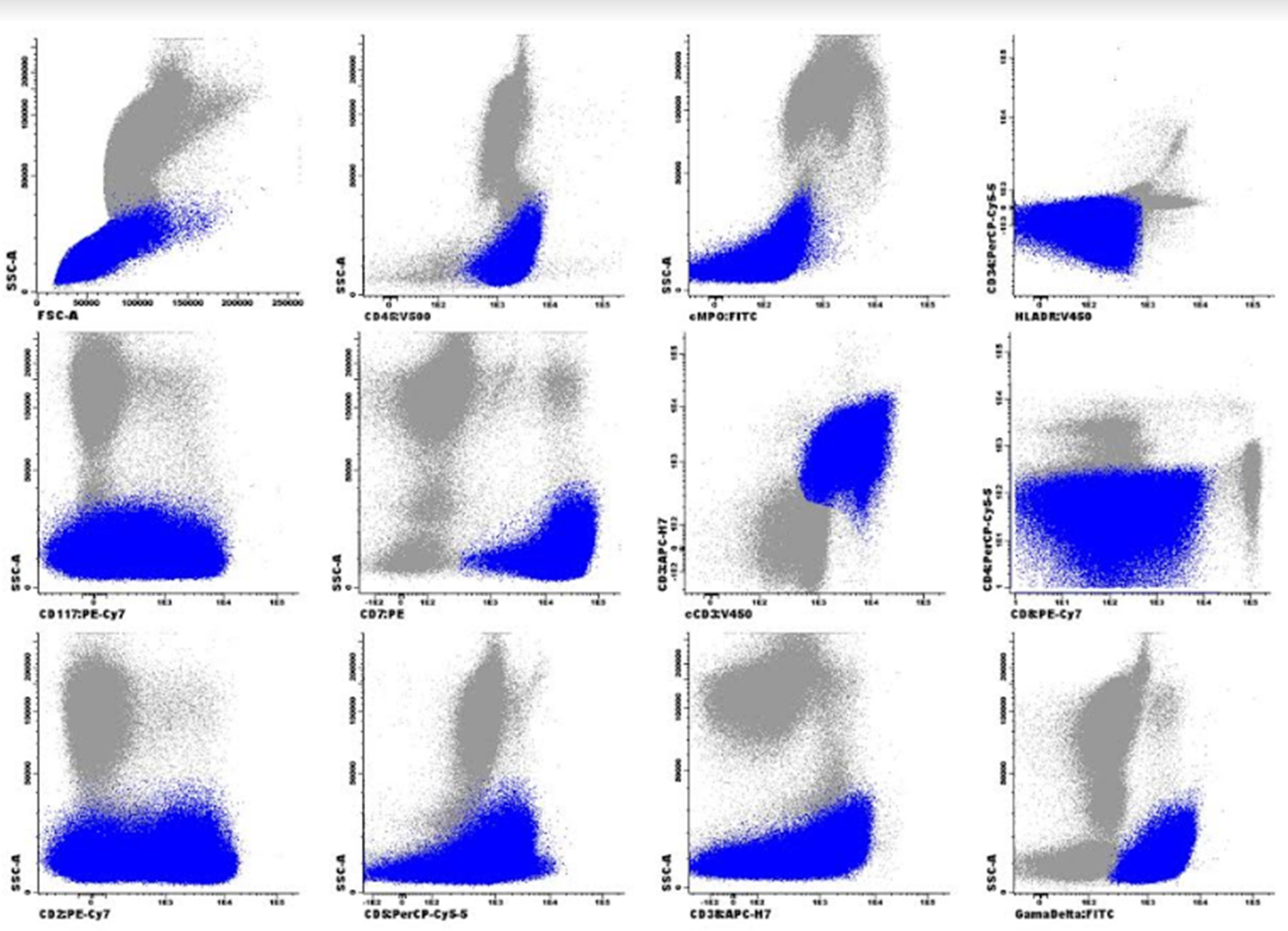
Despite CD117 expression, which is associated with FLT3-ITD mutation and consequently worse prognosis,4 FLT3-ITD analysis of the patient was negative, and the karyotype showed no growth. 7+3 protocol was performed with induction failure, replaced by two IDA-FLAG cycles. She was subjected, in June 2018, to HSCT, non-myeloablative by cardiotoxicity (FluCy + TBI). Evaluations of D+30, D+180 and D+360 post HSCT showed morphological remission, negative bone marrow minimal residual disease (MRD) with a limit of detection of 1 × 10−5 events, and complete chimerism. In October 2019, the patient attended the review consultation complaining of tiredness. In physical examination, she presented pallor, without skin lesions, and without palpable visceromegaly or adenomegaly. Blood count showed leukocytes: 308.76 × 109/L, Hb: 9.8g/dL and platelets: 94 × 109/L with presence of immature cells. Myelogram identified hypercellularity, infiltration by pleomorphic blasts with loose chromatin, evident nucleolus, agranular and high nucleus-cytoplasm relationship compatible with Acute Leukemia (Figures 2 and 4). There were no cytogenetic changes in karyotype. Immunophenotyping showed 84.0% of lymphoid T-cells (cCD3+, sCD3+, CD99+weak, CD7+strong, CD5+weak, CD2+weak heterogeneous, CD38+heterogeneous, CD8+weak partial in 20% of population, γδ TCR and CD45+) with heterogeneous CD117 antigen expression without other stem cell or myeloid markers (CD34, HLA-DR, CD13, CD33). These cells were negative for cMPO, CD19, CD10, nTdT, CD56, CD123, CD1a, CD44, CD45RA, CD4 and αβ TCR.
Due to positivity to sCD3 and the presentation of γδ T-cell receptor (TCR), it could be γδ T lymphoma. However, the absence of hepatosplenomegaly, marked leukocytosis, and the presence of T antigens with CD117 expression corroborates a diagnosis of T-strain Acute Lymphoid Leukemia (T-ALL). The possibility of mixed phenotype was excluded due to the absence of MPO. Expression of sCD3, partial and weak expression of CD8 and lack of other myeloid (CD11b, CD13, CD33) or stem cell (CD34, HLADR) markers excluded the classification of Early T Leukemia. Thus, in this case, according to clinical findings and other laboratory tests, this immunophenotype was suggestive of T-ALL with expression of CD117 and γδ TCR. The patient was treated with an increased Hyper-CVAD protocol, presenting morphological remission and immunophenotyping with absence of anomalous cells after the first cycle. Due to severe infectious complications (candidemia with systemic involvement requiring surgery for heart valvuloplasty) and worsening of the general state, the patient chose not to follow treatment, presenting a new recurrence in May 2020. The patient underwent palliative treatment, and died in 3 months.
DiscussionWe present a case of γδ T-ALL in an adult female patient, after HSCT with initial diagnosis of AML. The differential diagnosis of this case includes cutaneous and mucous T-cell lymphoma in the leukemic phase, γδ-HSTCL, Early T acute lymphoblastic leukemia (ETP-ALL), and γδ T-cell large granular lymphocytic leukemia (γδ-TLGLL).
Peripheral T-cell lymphoma can affect skin and mucosal regions, such as the nasopharynx, lung and gastrointestinal tract, different from this case, which had no evidence of mucocutaneous infiltration.5
γδ-HSTCL is a rare type of T-cell lymphoma with extra nodal and systemic involvement. Some studies support that clinical data such as splenomegaly is the most consistent clinical finding, hepatomegaly occurs in about 50% of patients and the majority of patients present with bone marrow involvement.6 In this case, there was medullary infiltration, but without evidence of splenomegaly, besides the fact that the immunophenotype evaluation presented markers of immaturity, discarding this diagnostic possibility.
γδ-TLGLL would be a possible diagnosis due to clonal proliferation of T cells with weak and partial CD8 expression; however, in general this disease, presents with an indolent clinical course, rarely associated with cytopenia, besides the peculiar morphology of cells with large volume and cytoplasmic granulation. 7
Bone marrow is affected in almost all cases of T-ALL, and the mediastinal or thymic involvement is common. Patients tend to have a high count of leukocytes, lymphadenopathy, and hepatosplenomegaly. Morphologically, lymphoblasts have an intermediate size and with delicate chromatin, small nucleolus and scarce cytoplasm. Immunophenotypically, they are generally positive for CD1a, CD2, CD3, CD5, CD7, CD10 surface and/or cytoplasmic, CD34, CD45, and TdT, and are negative for B-cell and myeloid antigens. In these cases, the expression levels of αβ TCR or γδ TCR in association with different stages of differentiation are rarely reported.8
ETP-ALL/LBL expresses markers of stem cells or myeloid lineage and is a high-risk T-ALL, with worse outcomes, especially in adults.9 Blasts usually express CD7, often weak CD5 (<75% positive cells), in the absence of CD1a and CD8, and positivity for ≥1 markers of stem cell or myeloid lineage (i.e., CD34, CD117, HLA-DR, CD13, CD33, CD11b, CD65).2 Therefore, this diagnosis was also excluded due to the expression of CD8, as well as the absence of other myeloid markers, despite the expression of CD117.
The γδ T-ALL is a rare variant of T-line lymphoblastic leukemia/lymphoma, and when compared with αβ T-ALL, it tends to present a more severe anemic picture in children, whereas in adults there is a high leukocyte count and splenomegaly.3
The diagnosis of γδ T-ALL is based on immunophenotypic features, so we started defining the lineage of the abnormal cells, positive for sCD3, CD7, cCD3 and negative for cMPO and cCD79a. Thus, these were definitely T cells. Then, we clarified the maturation stage based on expression of sCD3 and CD1a (early, thymic or mature T-ALL). T-ALL cells in this case were classified as mature medullary T-ALL (CD1a−, sCD3+) with γδ TCR expression.10 The present case showed clinical and immunophenotyping findings that led to the diagnosis of a “de novo” ALL (dn-ALL). We know that other diagnostic possibilities should be explored, such as therapy-related acute lymphoblastic leukemia (t-ALL) and lineage switch relapse. Data suggest that t-ALL are associated with deletions of chromosomes 5, 7, 11, 13, 17, and 20, as well as trisomy 8 and higher rates of poor-risk cytogenetic features such as MLL1 rearrangement.11,12 The same higher rates of poor-risk cytogenetic features such as MLL1 rearrangement can be seen in lineage switching from B-ALL.13 Unfortunately MLLr analysis was not available for the patient at the time. Lineage switching would be possible but there were no cytogenetic signatures to link in the original diagnosis.
The findings in this case suggest that the appropriate diagnosis was γδ T-ALL, this entity may represent a subcategory of acute lymphoblastic leukemia with slightly distinct clinical and laboratory characteristics with high risk group for induction failure and decreased survival.14,15 Molecular and cytogenetic studies can help in the definition of the diagnosis, but further studies investigating the role of genetics in γδ T-ALL are needed.