B cell acute lymphoblastic leukemia-lymphoma (B-ALL) accounts for approximately 75% of ALL cases and is observed in children and adults. Recent advances in disease diagnosis, stratification and prognostication have led to a better characterization of different subgroups of ALL. Notwithstanding the significant improvement in the complete remission rate of B-ALL, patients with minimal residual disease (MRD) and relapsed/refractory (R/R) settings suffer from poor outcomes. Hypothesis: However, novel therapies, such as agents targeting tyrosine kinases or the CD20 molecule, combination therapies and improved supportive care, have changed the treatment landscape of B-ALL.
Method and resultsMeanwhile, blinatumomab has been FDA-approved for MRD-positive or R/R B-ALL patients. Blinatumomab is a bispecific T cell engager containing the CD3 and CD19 that recognize domains redirecting cytotoxic T cells to lyse B cells. Promising outcomes, including long-term overall survival and improved MRD-negative response rates, have been reported in patients who received this drug. Adding blinatumomab to new ALL regimens seems promising for achieving better outcomes in poor prognosis B-ALL patients. Nevertheless, the neurotoxicity and cytokine release syndrome are the two major adverse events following the blinatumomab therapy.
ConclusionThis review summarizes the function and effectiveness of blinatumomab in R/R and MRD positive B-ALL patients. Furthermore, blinatumomab's positive and negative aspects as a novel therapy for B-ALL patients have been briefly discussed.
Enhancing conventional chemotherapy with targeted therapies increased the complete remission (CR) rate and the long-term survival rate of newly diagnosed acute lymphoblastic leukemia (ALL) patients to 85–90% and 40–45%, respectively. However, about one-third of patients at standard risk and two-thirds of high-risk patients experience relapse.1,2 Relapsed/ Refractory (R/R) B cell ALL (B-ALL) results in worse CR and long-term survival outcomes.1,3,4 Numerous studies are underway to improve R/R ALL outcomes. Applying advanced methods, such as real-time quantitative-PCR (q-PCR), multicolor flow cytometry (MFC) and next-generation sequencing (NGS) for accurate diagnosis of minimal residual disease (MRD), has a significant impact on managing patients' recurrence and improving their survival. Evaluating the MRD is the most beneficial tool in the relapsed ALL diagnosis. The presence of more than 0.01% of the detectable blast percentage is considered positive MRD.5-9
As mentioned above, the combination of targeted therapies, such as tyrosine kinase inhibitors (TKIs) and anti-CD20-antibodies, with conventional chemotherapy significantly impacted the ALL outcome.10-14 The CD19, a type I transmembrane (TM) glycoprotein, is exclusively expressed in the B lineage, including both normal B cells and B cell leukemia and lymphoma.15,16 Blinatumomab (Blincyto, Amgen), a newly emerged CD3-CD19 bispecific T cell engager (BiTE),17 has two recombinant single-chain variable fragments that temporarily link CD3+ T cells and CD19+ B cells, leading to the T cell-mediated lysis of neoplastic B cells.18 This article reviewed the effects of blinatumomab on B-ALL management.
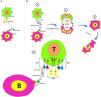
Blinatumomab mediates B cell apoptosis via activated T-cellsBlinatumomab is an anti-CD3/CD19 BiTE antibody with two recombinant single-chain variable fragments that temporarily link CD3+ T cells (ε chain in CD3) and CD19+ B cells (CD19 chain), leading to T cell-mediated lysis of B cells.7,12 Since the CD19 is a pan B cell antigen, the anti-CD19 variable fragment of blinatumomab targets this antigen and overcomes the B cell lineage leukemia/lymphoma. The transient engagement of tumor cells occurs by linking the drug to the CD3 of T cells. After the bridge-like linking of blinatumomab between B cells and T cells, a signaling pathway is triggered, leading to the T cell activation and release of granzymes and perforins into the immunological synapse. At the end of these interactions, tumor cell destruction occurs by the activation of caspases and apoptosis (Figure 1).13
Blinatumomab's mechanism of action. a. Interaction between anti-CD19 and CD19 on B cells and anti-CD3 and CD3 on T cells. b. The main components of the blinatumomab (two recombinant single-chain variable fragments of anti-CD3 and anti-CD19) and its mechanism of action; 1. Transient engagement of B cells and T cells by blinatumomab; 1.1. Anti-CD3 links ε chain of CD3 on T-cells anti-CD19 connects CD19 on B cells; 2. Immunological synapse formation and releasing of serine proteases (such as perforins and granzymes) by activated T cells, and; 3. B cell apoptosis.
Eradicating MRD is one of the most critical goals in disease management and outcomes improvement in ALL patients. The MRD is incapable of detection by routine cytomorphology and is only detected by more sensitive methods, such as flow cytometry, PCR techniques and NGS.1,2,19 Various clinical trials investigated the effect of blinatumomab on MRD+ B-ALL patients. In a study by Topp et al., blinatumomab (15 µg/day, 4 weeks on and 2 weeks off) was administered to 21 B-ALL patients with MRD ≥ 1 × 10−4. Among the evaluated patients, sixteen (80%) achieved negative MRD after administrating the first course of blinatumomab.3 Eligible patients with suitable donors were allowed for allogeneic hematopoietic stem cell transplantation (allo-HSCT) after the first course of blinatumomab therapy. Thirty-three months of follow-up disclosed the efficacy of a 61% overall relapse-free survival (RFS) rate (65% in patients who underwent HSCT and 60% in patients who did not undergo HSCT).4 Moreover, 50% of the 20 mentioned patients remained in CR at the end of the analysis at 50.8 months (56% of the patients had undergone HSCT and 45% had not).5
Gökbuget et al. followed the earlier study with 4 courses of blinatumomab (15 µg/day, 4 weeks on and 2 weeks off) in B-ALL patients in hematologic CR and with recurrent or persistent MRD (≥ 10−3) after ≥ 3 courses of intensive chemotherapy. Of 116 patients who received blinatumomab and were evaluated for MRD, 78% achieved negative MRD after the first course of blinatumomab and approximately 2% achieved negative MRD after the second course, but other patients did not achieve MRD negativity even after courses 3 or 4.6 The second study by the same group investigated the survival and HSCT in 110 ph+ B-ALL patients; they reported a median relapse-free survival (RFS) and overall survival (OS) of 18.9 months and 36.5 months, respectively.20
Remarkably, patients achieving negative MRD after the first course of blinatumomab demonstrated significantly superior survival outcomes than MRD+ patients; the median RFS was 23.5 vs. 5.6 months (p = 0.002) and median OS was 38.8 vs. 12.4 months (p = 0.002) in MRD-positive and MRD-negative patients, respectively. Thus, these observations revealed a privilege in altering the positive MRD to the negative one. Since this study showed a superior effect of blinatumomab on RFS and OS in the first CR, compared to the second or third ones, administering it in the first CR was suggested to eradicate the MRD. Among 110 ph-positive B-ALL patients, 67% (74/110) underwent allo-HSCT and 49% (36/74) remained in CR. Interestingly, 25% (9/36) of ph-ALL patients who did not receive chemotherapy or HSCT remained in CR after blinatumomab.21 Blinatumomab received FDA approval for B-ALL patients in CR with MRD ≥ 10−3 (0.1%) in 2018.7
The effect of blinatumomab on the survival of Ph-positive B-ALL patients with recurrent or persistent MRD of ≥ 10−4 was evaluated by Richard-Carpentier et al., who showed no significant difference in RFS or OS between Ph-positive and Ph-negative patients in two years of follow-up.8
Optimal MRD for using blinatumomabAs mentioned earlier, blinatumomab was approved by the FDA for an MRD ≥ 10−3 according to the Gökbuget et al. study.21 However, the problematic feature of MRD thresholds, absence of standardization of the MRD level for clinical decisions and advent of high sensitivity methods for MRD detection led to using various MRD thresholds in different clinical trials.3,8 For example, the FDA-approved-clonoSEQ-NGS assays support MRD detection at 10−6.7 On the other hand, more technological advances in the future are likely to increase the ability to detect very low levels of MRD. Therefore, future studies should be conducted to clarify if patients with very low levels of MRD benefit from blinatumomab or not.
Performing HSCT after MRD-negativity following blinatumomab or not?The allo-HSCT is recommended for ALL patients who do not respond to induction therapy.9,10 However, it is not yet clear whether positive MRD patients who show undetectable MRD after blinatumomab therapy will benefit from the HSCT or not. For example, the BLAST study showed that 25% of non-transplanted patients and 49% of transplanted patients remained in CR. Therefore, all blinatumomab respondents did not require HSCT.7,21 However, these trials were not performed to evaluate the impact of HSCT after blinatumomab.
For those MRD patients who respond to blinatumomab, but are not suitable candidates for transplantation, it is necessary to determine the optimal number of blinatumomab cycles and evaluate the demand for combination therapy. In the case of HSCT with blinatumomab administration, the effect of blinatumomab in post-HSCT maintenance should be investigated.11
Real-world data of B-ALL patients treated with blinatumomabThe advent and utilization of blinatumomab is evidence of shifting the old paradigm to the emerging plans for better B-ALL management (Table 1). A B-ALL cohort with 239 patients (relapsed refractory, n = 227, and MRD positive, n = 12) who received blinatumomab therapy evaluated the safety and efficacy of this approach in the real-world context. Before blinatumomab initiation, 26% (n = 61) patients had received ≥ 3 previous therapies and 19% (n = 46) had undergone HSCT. The CR/CRi rate (CR with incomplete hematologic recovery) was 65% in RR patients. Among MRD-positive patients who received blinatumomab, 75% achieved MRD negativity. RR patients who received blinatumomab showed median RFS and OS of 32 months and 13 months, respectively. In positive MRD patients, the median RFS and OS were not achieved. Neurotoxicity, hepatotoxicity and cytokine release syndrome (CRS) with grade ≥ 3 were reported in 7%, 10% and 3% of patients, respectively. The allo-HSCT, as consolidation therapy in patients with CR/Cri, retained its favorable prognostic effect for OS (p = 0.04).13,14
Clinical trials of blinatumomab in R/R B-ALL.
| Study | Results | Ref |
|---|---|---|
| Phase II trial |
| 22 |
| Phase II trial |
| 23 |
| Phase Ib |
| 24 |
| Phase II |
| 25 |
| Phase II |
| 26 |
| Phase III |
| 27 |
| Phase II |
| 28 |
| Randomized phase III |
| 29 |
| Retrospective multicenter study |
| 30 |
CR/CRh, Complete Remission/Complete Remission with Partial Hematologic Recovery; MRD, Minimal Residual Disease; RFS, Relapse-Free Survival; OS, Overall Survival; R/R B-cell precursor ALL, Relapsed/Refractory B-Cell Precursor Acute Lymphoblastic Leukemia; R/R Phþ ALL, Relapsed/Refractory Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia; SOC, Standard Of Care; allo-HSCT, Allogeneic-Hematopoietic Stem Cell Transplantation.
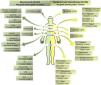
Approximately 98.5% of patients receiving blinatumomab will show adverse drug reactions, including 62% presenting serious complications and 19%, fatal adverse effects, with 12.5% requiring immediate treatment cessation.15 Blinatumomab side effects and its standard of care chemotherapy (with or without anthracycline chemotherapy, high-dose methotrexate, high-dose cytarabine (HiDAC), or clofarabine regimens) are displayed in Figure 2. The most significant adverse drug reactions are neurotoxicity and cytokine release syndrome.
Unexpected neurological adverse events and their managementNeurological adverse events are one of the major causes of the blinatumomab therapy termination.17 Manifestations of neurotoxicity vary from mild symptoms, such as headache, confusion, tremor, or disorientation, to serious clinical symptoms, such as seizures, aphasia, or stupor. The major reason for blinatumomab-induced neurologic toxicity is still ambiguous.
A probable two-step model has been suggested for a comprehensive understanding of the blinatumomab-induced neurotoxicity mechanism. First, blinatumomab induces T-cell reorganization from the peripheral blood to the perivascular space in a B-cell-independent manner. Subsequently, in a B-cell-dependent manner, it induces T-cell activation and cytokine release. In sequence, the released cytokines cause neurotoxicity.18,31 In neurotoxicity grades 1 to 2, supportive treatments with intravenous fluids, anti-inflammatory agents and respiratory support therapies are suggested. Additionally, steroid therapy with dexamethasone 8 mg/ every 8 hours should be started as a priority to avoid progression to a higher grade. In the case of symptom progression, blinatumomab therapy discontinuation is necessary. In the case of grade 3 neurotoxicity, blinatumomab therapy must be ceased to reduce the neurotoxicity grade ≤ 1 for at least 3 days. Once the patient's condition becomes stable, blinatumomab can be resumed at the initial dose and increased after 7 days. For patients who present continuous grade 3 neurotoxicity for more than 7 days, or reach grade 4 neurotoxicity, following the re-administration of blinatumomab, permanent drug termination is recommended.32 In these cases, dexamethasone at 8mg., every 8 hours for 3 days, and a tapering dose in 4 days is recommended. Since the seizure incidence rate is very low (< 1%), prophylaxis is only conducted in particular contexts.15 Moreover, further central nervous system (CNS) examinations are needed to rule out other probable neurological diseases.
Unexpected cytokine release syndrome and its managementAlthough immunotherapy adventures profoundly affect managing hematological malignancies, its related toxicity has overshadowed its valuable benefits. The CRS is one of the most crucial neurological adverse effects following T cell-based therapies, such as blinatumomab. Antigen-antibody interaction, cytotoxic T cells activation and subsequent monocytes and macrophages activation, which are all induced by blinatumomab, are the major causes of massive inflammatory cytokine release, systemic inflammatory response syndrome and CRS.31 In this regard, increased levels of interferon gamma (IFN-γ), interleukin 6 (IL-6) and interleukin 10 (IL-10) have been reported in ALL patients who received blinatumomab.33 Cytokine elevation could be associated with both the toxicity and efficacy of blinatumomab. The CRS could present a broad spectrum of clinical manifestations from mild to severe symptoms, including fever, flu-like symptoms, hemodynamic instability, coagulopathy and capillary leak syndrome, resulting in multi-organ system failure.22
A phase III clinical trial has reported a relative incidence rate of 16% and 5% in CRS with any grade and CRS with grade ≥ 3, respectively.34 Treatment discontinuation and interruption due to CRS occurred in 1% and 5%, respectively.15 On the other hand, the CRS rate was lower in the MRD positive group than in the R/R patients due to the difference in tumor burden and elevated cytokines release in the two groups.20 Considering the CRS incidence and severity are considerable issues in starting the blinatumomab therapy, prophylactic treatment, cytoreduction, pre-phase dexamethasone, dose modification and dose interruption are the assessments for such a purpose.35
A corticosteroid is suitable supportive care to restrain blinatumomab-induced CRS. However, disrupting the T cell function and reducing the blinatumomab clinical benefit has remained a concern in the corticosteroid therapy. In light of IL-10, IL-6, and IFN-γ increase following the blinatumomab-related CRS, treatment options targeting these cytokines could address the CRS consequences.36 The IL-10 and IFN- γ are undesirable cytokines for targeting therapy because the IFN-γ likely plays a role in the blinatumomab efficacy and the IL-10 is a negative regulator of the immune system.37,38
The IL-6 blockade using tocilizumab provides promising results in controlling CRS in patients who received blinatumomab or chimeric antigen receptor T cells (CAR T cells).39,40 Tocilizumab received FDA approval in 2010 and is prescribed to treat rheumatoid arthritis, systemic juvenile idiopathic arthritis, polyarticular juvenile idiopathic arthritis, giant cell arteritis and CRS.41,42 Studies suggested that tocilizumab could ameliorate CRS symptoms without affecting the effectiveness of T cell-related therapies. Tocilizumab at dosages of 12 mg/kg and 8 mg/kg is prescribed for patients weighing less than or over 30kg.43 Anakinra, an IL-1 receptor antagonist, has shown remarkable effects in controlling CAR T-induced CRS and CAR-related encephalopathy syndrome.44 Anakinra or tocilizumab have not inhibited CAR T cell effectiveness; therefore, they are suitable for the prophylactic management of CRS.45 Since CRS pathophysiology mechanisms are similar in both blinatumomab and CAR T cell therapy, IL-1 antagonists may hinder blinatumomab-induced CRS. Further experimental and clinical studies would be essential in understanding the efficacy of tocilizumab and anakinra in CRS prevention and treatment.
Resistance to blinatumomab therapyOne of the critical issues considered in blinatumomab therapy is the risk of resistance.7 As Table 2 shows, one possible mechanism of blinatumomab resistance is the loss of the target antigen.46 Treating mixed-lineage leukemia (MLL)-rearranged B-ALL patients with blinatumomab is another possible mechanism of blinatumomab resistance, which may switch lineage from ALL to AML.47-50
The mechanisms of resistance to blinatumomab therapy.
| Type of Resistance | Mechanism | Ref |
|---|---|---|
| Loss of T cell activity | Up-regulation of PDL-1 expression on leukemic cells and PD-1 expression on T cells contributes to T cell exhaustion.Up-regulation of Tim-3, and TIGIT expression in CD8+ T cells of non-responder patients and elevated expression of Galectin-9 (Tim-3 ligand), PD-L2 (PD-1 ligand) and CD155 (TIGIT ligand) on tumor cells.High regulatory T cell (Treg) frequency leads to suppression of T cell proliferation and reduction of anti-leukemic cytotoxicity. | 51,53,54 |
| Modification of the target antigen | Down-regulation CD19 expressionSuppressive microenvironment Disruption in CD19 membrane export | 55,56 |
PDL-1, Programmed death ligand- 1; PD-1, programmed cell death protein 1; Tim-3, T cell immunoglobulin and mucin domain 3; TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domains.
On the other hand, since the T cell activity is one of the important parameters in blinatumomab efficacy, decreasing the T cell activation could be a possible reason for the blinatumomab resistance. Evidence of this claim is the increased expression of T cell exhaustion markers PD1 and PD-L1 following blinatumomab therapy. Thus, the immune checkpoint inhibitors may influence the blinatumomab efficacy.51,52 The anti-leukemic response was increased in a 12-year-old patient with refractory ALL after combination therapy with blinatumomab and anti-PD-1 antibody. The blockade of the PD-1/PDL-1 pathway or CTLA-4 could enhance the T cell function and improve the blinatumomab efficacy in patients who expressed T cell exhaustion markers [51]. A multi-center phase I dose-escalation study evaluated the combination of blinatumomab plus nivolumab (anti-PD-1 monoclonal antibody) and ipilimumab (anti-CTLA-4 monoclonal antibody) in R/R CD19+ ALL patients. Their results revealed that the combination of blinatumomab and nivolumab/ipilimumab is feasible and tolerable. Among five patients, four achieved CR, whereas one developed extra-medullary relapse.51 Several clinical trials (such as NCT02879695, NCT04546399 and NCT03512405) were conducted to explore the effectiveness of combination therapy by blinatumomab and the immune checkpoint inhibitors in R/R ALL patients. Further studies would be required to prove the positive impacts of immune checkpoint inhibitors on overcoming blinatumomab resistance.
ConclusionAdvanced treatment strategies and novel drugs have improved the clinical outcome of B-cell ALL patients. For instance, tyrosine kinase inhibitors (target JAK/STAT and mTOR pathways), antibody drug-conjugate, bispecific antibodies and chimeric antigen receptor T cells are promising in achieving more favorable outcomes. However, the outcome in R/R ALL patients remains poor. The FDA has approved blinatumomab for R/R B-ALL and MRD+B-ALL. The poor prognosis ALL patients with some translocations, such as the TCF3/HLF-fusion and Philadelphia-positive or Philadelphia-like ALL are candidates to receive blinatumomab. single-agent blinatumomab and its combination with other therapies, such as TKIs have improved survival and long-term outcomes. Furthermore, combining checkpoint inhibitors (anti-PDL-1 and anti-CTLA-4 antibodies) with blinatumomab could enhance its effectiveness. Although blinatumomab administration is tolerable and safe, prevention and optimal management of blinatumomab-induced adverse reactions are considerable issues.
Data availability statementThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributionsB.H.K., S.P. and RM. were responsible for the conception and design and acquisition of data; H.G.N., M.M., drafting the article or revising it critically for important intellectual content, and; M.T.A., M.S. and M.D.G.H, methodology (lead), writing, review and editing. All authors revised the final approval of the version to be submitted for publication.