Chikungunya virus, an arbovirus that belongs to the Alphavirus genus of the Togaviridae family, causes a febrile illness accompanied by rash and arthralgia. It is estimated that during outbreaks, the prevalence of Chikungunya virus RNA in viremic blood donations varies between 0.4 and 2.1%; therefore, this virus may be transmitted by transfusion. In Brazil, Chikungunya virus has been claimed to cause extensive outbreaks, however, the seroprevalence of anti-Chikungunya virus IgG among Brazilian blood donors is unknown.
MethodsEight hundred and ninety-seven blood samples were collected from volunteer blood donors in two distant localities long after the Chikungunya virus first appeared in Brazil. In 2015, 442 samples were collected from the Hemotherapy Service of Macapá, Amapá in the northern Brazilian Amazon. To evaluate the dissemination course of the virus in Brazil, in 2016, 455 blood samples were collected from the southeastern region (Blood Center of Ribeirão Preto, Ribeirão Preto, São Paulo). All samples were tested for the presence of anti-Chikungunya virus IgG and viral RNA.
ResultsOne sample (0.2%) obtained from the Hemotherapy Center of Macapá tested positive for anti-Chikungunya virus IgG and no sample from the Blood Center of Ribeirão Preto was seroreactive to anti-Chikungunya virus IgG. All blood donations were Chikungunya virus RNA negative.
ConclusionsThis study, performed during 2015–2016, indicates that the transfusion risk of Chikungunya virus in this period was low. However, due to the constant advance of this virus in Brazil, further studies during outbreaks are needed to evaluate the presence of Chikungunya virus RNA in blood donations and the respective transfusion-transmission risk.
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus belonging to the Togaviridae family. Due to its similar antigenic properties with other important human alphaviruses such as O’nyong-nyong, Mayaro (MAYV) and Ross River viruses (RRV), CHIKV is additionally classified within the Semliki Forest serocomplex. Three CHIKV genotypes have been identified i.e. West African, East/Central/South African (ECSA) and Asiatic.1 CHIKV causes extensive outbreaks of acute febrile illness with arthralgia. Symptoms of infection may include arthritis, myalgia and/or maculopapular rash making the clinical presentation indistinguishable from Dengue (DENV) or Zika (ZIKV) fevers.2 In about 28% of cases, however, CHIKV infection may remain asymptomatic.3 The presence of an asymptomatic/oligosymptomatic course of CHIKV infection and the high pre-symptomatic viral load (approximately 108pfu/mL)4 suggest that the infection can represent a threat to blood transfusion safety. Although transfusion-transmission of CHIKV has not been documented, a recent report of transmission of RRV by erythrocyte components,5 demonstrates that transfusion transmission of CHIKV cannot be underestimated.
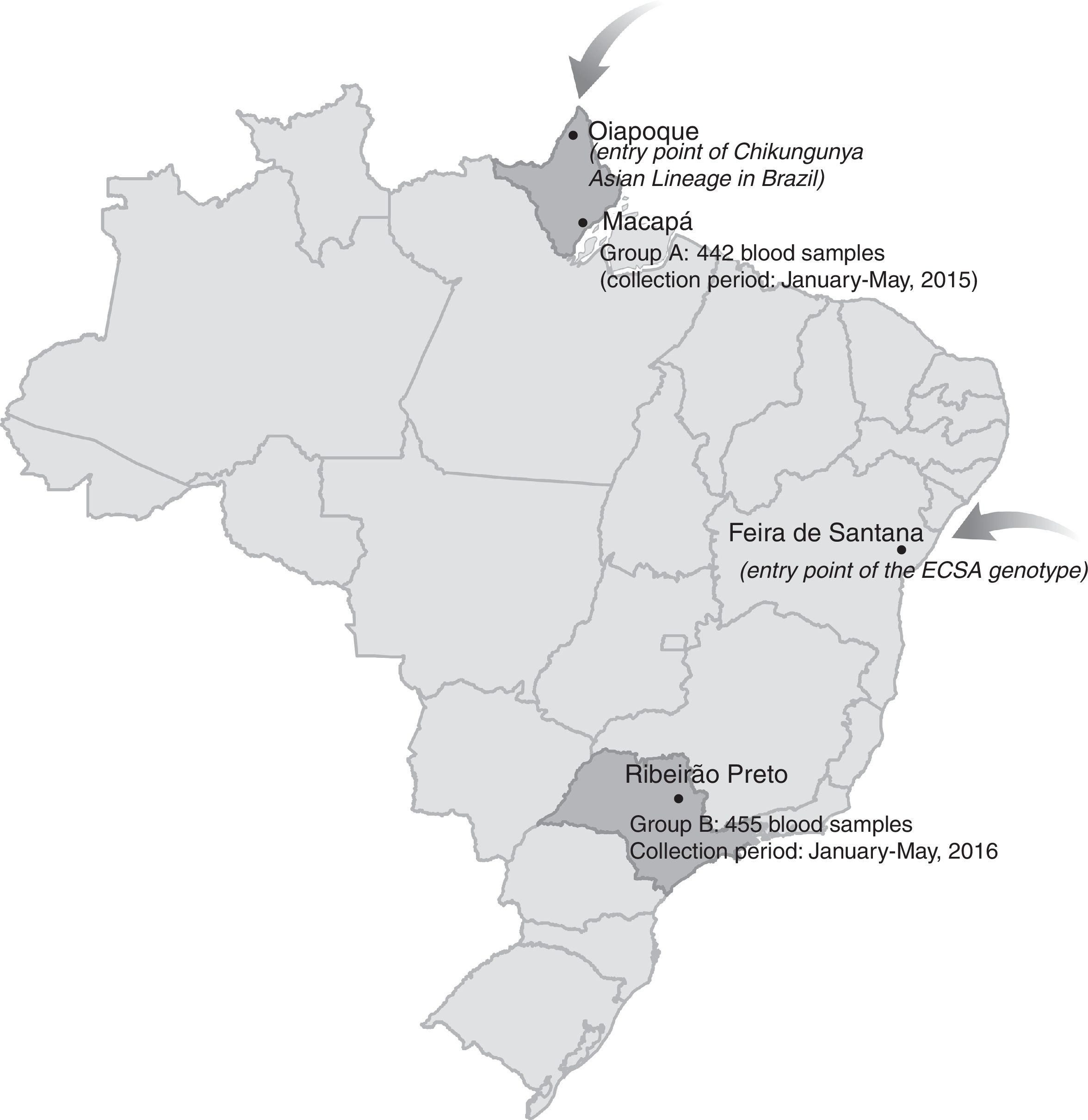
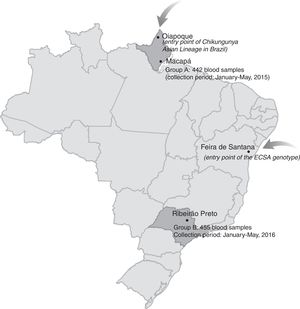
The first cases of autochthonous transmission of CHIKV in the Americas were reported in 2013 on Saint Martin Island in the Caribbean with the virus being rapidly disseminated to other islands and mainland South America.6 By mid-2014, CHIKV autochthonous transmission was confirmed in Brazil in two geographically distinct and distantly located regions. The Asian lineage was detected in the small town of Oiapoque (Amapá State, Northern Amazon) located on the French Guyana-Brazilian border, while the ECSA genotype was accidentally discovered in Feira de Santana (Bahia State, Northeast Brazil). The circulation of both genotypes in Brazil and in distantly located regions suggests independent routes of viral introduction.7
In Brazil, CHIKV has been claimed to cause extensive outbreaks; however, there is no study which evaluates the prevalence of anti-CHIKV IgG in blood donors. Therefore, the objective of this study was to perform an anti-CHIKV IgG serosurvey in Brazilian blood donors from two distinct geographic regions and at two different timepoints in order to evaluate the dissemination of CHIKV in Brazil and its transfusion risk. Initially, between January and May 2015, blood donors from Northern Brazil (entry point of CHIKV) were tested for anti-CHIKV IgG at the time of the introduction of the Asian lineage. In order to investigate whether CHIKV had reached Southern Brazil, blood donors of Ribeirão Preto, São Paulo State were tested for anti-CHIKV IgG during an extensive DENV-like outbreak one year later, between January and May 2016. The serosurvey is necessary because CHIKV seroprevalence and transfusion risk in Brazil are completely unknown and outbreaks have been reported in different geographic regions of the country.
MethodsPlasma samples were collected from blood donors in two different periods and from two distantly located regions in order to evaluate the dissemination of CHIKV in the country after its introduction in 2014–2015. Between January and May 2015, 442 blood samples were collected from the same number of blood donors (Group A) in the Blood Center of Amapá, Macapá (Hemocentro do Amapá – HEMOAP), Northern Brazil (Figure 1 and Table 1). This city is located close to the entry point of the CHIKV Asiatic lineage in Brazil (about 500km). Blood samples were collected during an extensive DENV-like outbreak.
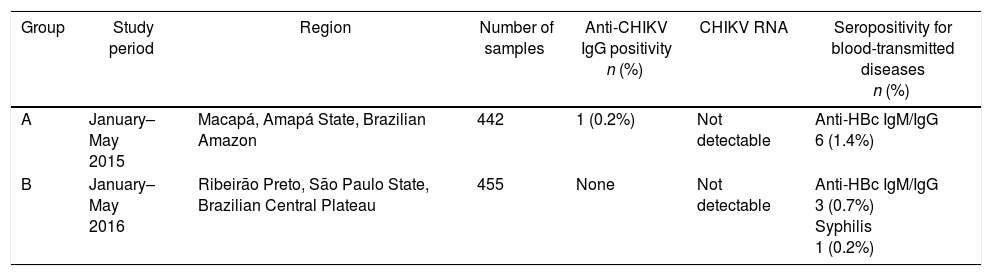
Demographical and serological characteristics of the blood donor groups investigated for anti-CHIKV IgG.
| Group | Study period | Region | Number of samples | Anti-CHIKV IgG positivity n (%) | CHIKV RNA | Seropositivity for blood-transmitted diseases n (%) |
|---|---|---|---|---|---|---|
| A | January–May 2015 | Macapá, Amapá State, Brazilian Amazon | 442 | 1 (0.2%) | Not detectable | Anti-HBc IgM/IgG 6 (1.4%) |
| B | January–May 2016 | Ribeirão Preto, São Paulo State, Brazilian Central Plateau | 455 | None | Not detectable | Anti-HBc IgM/IgG 3 (0.7%) Syphilis 1 (0.2%) |
Another 455 blood samples from the same number of blood donors (Group B) were collected one year later in the Blood Center of Ribeirão Preto during an extensive DENV-like outbreak (DENV and ZIKV co-circulation) (Figure 1 and Table 1). Ribeirão Preto is located in the northeastern region of São Paulo State (Southeastern Brazil).
Six milliliters of peripheral blood were collected in sterile EDTA tubes (Vacuette, Greiner Bio-One, Brazil). Plasma was separated by low speed centrifugation (290×g for 10min) and stored at −80°C until use. This project was approved by the Institutional Ethics Committee of Hospital das Clínicas of the Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo (process #2056/2015). All blood donors included in this study signed written informed consent forms and their epidemiological data was obtained during the sample collection.
Anti-CHIKV IgG was detected using the human anti-Chikungunya Virus IgG ELISA Kit (Abcam, Cambridge, MA, USA) following the manufacturer's instructions. All the controls (blank, cut off and positive) were included in duplicate. Additionally, a high-titer positive anti-CHIKV IgG plasma sample [>30 standard units (SU)] from a Brazilian traveller, who acquired the infection in Puerto Rico, was included in all plate readings in order to validate the serological reaction in regards to Brazilian samples. Results of optical density are represented as SU based on the manufacturer's instructions, following the formula: mean absorbance of the sample×10/mean cut-off. The manufacturer informed that the test has >90% specificity and sensitivity for the detection of anti-CHIKV IgG and does not cross-react with anti-DENV IgG.
In order to see if asymptomatic viremia was present in blood donors, all samples were additionally tested for CHIKV RNA by a previously described TaqMan® real-time molecular platform.8 The estimated analytical sensitivity of the test is 0.68copies/reaction (95% confidence interval, regression analysis as implied by the Probit algorithm). The sensitivity of the test was assessed by the application of a quantification curve based on a 10-fold dilution of a cloned 68-base pair CHIKV fragment using a TOPO® TA Cloning Vector (ThermoFisher Scientific, São Paulo, Brazil).
Reverse transcription was performed as a separate reaction using the High Capacity cDNA Reverse Transcription Kit (Thermofisher Scientific, São Paulo, Brazil) and 500nM of CHIKV specific reverse primer. Quantification of viral load was achieved in duplicate using the TaqMan® real-time polymerase chain reaction (PCR) mastermix (Thermofisher Scientific, São Paulo, Brazil), 300nM of specific primer and 150nM of the probe. Amplification was carried out in an Applied Biosystems 7500 cycler (Thermo Fisher Scientific, Waltham, MA, USA) using a standard amplification protocol. Precautions to avoid contamination were strictly adhered to.
ResultsOf 442 samples obtained from blood donors from Macapá (Group A), one was seroreactive to anti-CHIKV IgG (0.2%; 12 SU) (Table 1). Additionally, none of the blood donors were seroreactive to HIV-1/2, HCV, HTLV-1/2, American trypanosomiasis (Chagas disease) or Treponema pallidum, whilst six were positive for anti-HBc IgM/IgG (n=6/442; 1.4%) (Table 1). The positive anti-CHIKV IgG sample was from a young (28-year old) sporadic blood donor living in Macapá. At the time of the donation, he denied rash, joint pain, fever or any virally related symptom. He also denied traveling to other Brazilian states or abroad. All of the tested samples were negative for CHIKV RNA as confirmed by TaqMan® real-time PCR.
No seroreactivity to anti-CHIKV IgG was observed in the 455 blood samples obtained from blood donors from Ribeirão Preto (Group B, Table 1). These samples were similarly negative for CHIKV RNA by TaqMan® real-time PCR. Three donors (n=3/455; 0.7%) were seroreactive to anti-HBc IgM/IgG, one to Treponema pallidum (n=1/455; 0.2%; confirmed by the Rapid Plasma Reagin (RPR) test and immunofluorescence for anti-treponemal IgG) and no one demonstrated seropositivity for HIV-1/2, HCV, HTLV-1/2 or American trypanosomiasis (Table 1).
DiscussionThis study was performed in blood donors from two different regions of Brazil (the north and the southeast) and very low/no seropositivity for anti-CHIKV IgG was observed in both, i.e. 0.2% in Macapá (first half of 2015) and 0.0% in Ribeirão Preto (first half of 2016). As there was only one positive participant, it is not impossible to discard a serological cross-reactivity of the diagnostic kit with anti-MAYV antibodies. MAYV is an alphavirus that is closely related to CHIKV; both belong to the Semiliki Forest serogroup. However, in comparison to CHIKV, MAYV is endemic in Brazil and circulates extensively among the population of the Brazilian Amazon including Macapá.9 Another possibility is that the reactive sample may be a false-positive result. As the manufacturer specifies >90% specificity for anti-CHIKV IgG detection, it is theoretically possible that some samples (<10%) may be false positives. In any case, as the studied blood donor groups demonstrate very low/no seropositivity for anti-CHIKV IgG, it is important to stress that they were not greatly exposed to CHIKV infection and the transfusion-transmission of CHIKV in the studied period can be considered low.
Autochthonous transmission of CHIKV in Brazil with a high number of confirmed cases has been reported since 2014 in two distantly located regions: Bahia State (Northeastern Brazil) and Oiapoque, Amapá State in the Brazilian Amazon.7 It is possible that at the beginning of this survey, CHIKV probably had barely reached Macapá, thus corroborating these results. This is reinforced by epidemiological data from the Municipal Epidemiological Surveillance Agency of Amapá for this period, as between January and May 2015 no CHIKV cases were reported in Macapá. However, in some municipalities near Macapá, such as Oiapoque (924 notified cases), Porto Grande (eight notified cases) and Santana (two cases) there was significant CHIKV activity during the study period. It is possible that the blood donor who was positive for anti-CHIKV IgG had traveled to a nearby town and developed asymptomatic or subclinical CHIKV infection that was later diagnosed in this study.
Similarly, no CHIKV circulation was observed among blood donors of Ribeirão Preto during a large DENV/ZIKV outbreak in 2016. Probably this is due to the fact that although CHIKV had reached Ribeirão Preto (for more information see Supplement 1) during its dissemination throughout Brazil it did not spread widely in this region. During 2016, a relatively low prevalence of anti-CHIKV IgG (7.4%) was also registered in large urban centers such as Salvador, Bahia in different study groups (microcephalic and non-microcephalc pregnancies, HIV-infected patients, tuberculosis patients and university staff) contrasting to the high anti-ZIKV IgG prevalence (63.3%) obtained in the same period.10 A moderate prevalence of anti-CHIKV IgG (18.3%) was registered in randomly selected individuals from a rural community near Feira de Santana, Bahia State.11 These data demonstrate that the epidemic of CHIKV has been occurring more locally and with low endemicity largely contrasting to the ZIKV outbreak in Brazil, which reached national proportions. This is supported by the current seroprevalence study of anti-CHIKV IgG in volunteer blood donors in Ribeirão Preto, where the reported seroprevalence was 0.0% with 141 notified cases and nine confirmed for the period of January to May 2016 according to the Municipal Epidemiology Surveillance Agency (http://www.saude.ribeiraopreto.sp.gov.br/ssaude/pdf/dengue_balanco.pdf). By this time in the city, there was a large mixed DENV/ZIKV outbreak12 which demonstrates that CHIKV probably has different epidemiologic characteristics to ZIKV and DENV.
The results demonstrate the absence or delay of ongoing CHIKV outbreaks in the tested geographical regions. Therefore, we suppose that if this serologic survey was performed in both regions during 2017–2018, we would detect seropositive blood donors as previously reported in other countries with a history of CHIKV outbreaks.13 This is especially valid for Macapá, which is close to the point of entry of the CHIKV Asian lineage, however, different technical difficulties including lack of human resources for sample collection and difficulties for the sample logistics from this very remote part of Brazil precluded such an evaluation. Future nationwide studies are needed to evaluate the dynamics of CHIKV infection and its seroprevalence in blood donors in the most affected Brazilian states.
The results of this study also demonstrate that blood donors and probably, the general population of Ribeirão Preto, is currently immunologically naïve to CHIKV. Considering that herd immunity is an important protection factor, this population can be considered highly vulnerable to CHIKV outbreaks. Since there is growing data for severe complications due to CHIKV infection in Brazil14 active measures should be undertaken to avoid CHIKV outbreaks and further dissemination of this virus.
ConclusionsIn short, this serologic study, performed during 2015–2016, is probably the largest serological survey evaluating the prevalence of CHIKV in blood donors in Brazil. The large proportions of negative results observed in both regions are probably the result of the slow expansion of CHIKV in Brazil and indicates that larger surveys involving more Brazilian states should be carried out later, when the CHIKV has spread fully to these regions. The dissemination of CHIKV in Brazil is ongoing and further serologic and molecular surveys should be performed to evaluate the epidemiological situation of this emerging virus in blood donors.
Financial supportThis study was supported by the Fundação de Amparo e Pesquisa do Estado de São Paulo-FAPESP, Brazil (Grant № 2009/16623-1, CTC-2013/081352, and INCTC-2008/57.877-3), and the Conselho Nacional do Desenvolvimento Científico e Tecnológico, Brazil (INCTC-573.754/2008-0).
Conflict of interestThe authors declare no conflicts of interest.
We are grateful to Vanderléia Barbaro Valente, MSc for giving us the opportunity to use the infrastructure of the Laboratory of Serology, Blood Center of Ribeirão Preto. We are also grateful to Sandra Navarro Bresciani for the artwork.