Several studies have demonstrated that platelet counts in Helicobacter pylori-positive patients with chronic idiopathic thrombocytopenic purpura improved significantly after successful eradication of the infection. However, depending of the geographical region of the study the results have been highly divergent.
ObjectiveThe purpose of this study was to evaluate the effect of H. pylori eradication therapy on platelet count in a cohort of chronic idiopathic thrombocytopenic purpura patients from northeastern Brazil.
MethodH. pylori status was determined in 28 chronic idiopathic thrombocytopenic purpura patients using the rapid urease test and histology. H. pylori-positive patients received standard triple therapy for one week. The effect of the eradication therapy was evaluated using the 13C-urea breath test two to three months after treatment.
ResultsThe prevalence of H. pylori infection was similar to that found in the general population. Twenty-two patients (78.5%) were H. pylori-positive. Fifteen were treated, 13 (86%) of whom successfully. At six months, 4/13 (30%) displayed increased platelet counts, which remained throughout follow-up (12 months). Platelet response was not associated to mean baseline platelet count, duration of chronic idiopathic thrombocytopenic purpura, gender, age, previous use of medication, or splenectomy.
ConclusionsH. pylori eradication therapy showed relatively low platelet recovery rates, comparable with previous studies from southeastern Brazil. The effect of H. pylori eradication on platelet counts remained after one year of follow-up suggesting that treating H. pylori infection might be worthwhile in a subset of chronic idiopathic thrombocytopenic purpura patients.
Helicobacter pylori, a gram-negative microorganism first isolated by Warren & Marshall in 1984, colonizes the human stomach and may cause type B gastritis and peptic ulcers. Colonization of the stomach by H. pylori is associated with increased risk of gastric cancer,1 and a number of other non-gut-related disorders, such as coronary disease2 and autoimmune diseases including autoimmune thyroiditis3 and chronic idiopathic thrombocytopenic purpura (cITP).4
cITP is a poorly understood acquired hemorrhagic disease which involves the destruction of platelets in the reticuloendothelial system induced by anti-platelet antibodies.5 To date an effective and safe treatment for cITP has not been established. cITP treatment has been restricted to therapies with the potential of causing significant toxicity and risks including immunosuppressive agents, such as corticosteroids, intravenous immunoglobulin therapy (IVIg), anti-D immunoglobulin (anti-D), rituximab and salvage splenectomy. Furthermore, 20–30% of cITP patients are resistant to these therapies.6
After the discovery by Gasbarrini that platelet counts in H. pylori-positive cITP patients improved significantly after successful eradication of the infection,4 several authors from different geographical regions have evaluated the effect of H. pylori eradication therapy on platelet counts in this patient population. However, results have been highly variable (0–100%).7
The highest response rates (>50%) are of cohorts in Italy,8 Japan,9 Korea10 and Colombia.11 On the other hand, in a study from Spain, only 13% experienced a significant increase in platelet counts as a result of H. pylori eradication,12 whereas a study from the United States found no difference between groups.13
Differences in the genetic background of the host and in the virulence of H. pylori strains may explain the discrepancies observed in studies on H. pylori eradication therapy in cITP patients. This bacterium has several virulence genes, showing a high variability of distribution with the most important being the vacuolating cytotoxin A gene (VacA) and cytotoxin-associated gene A (cagA). The cagA gene is part of a 40kb cluster of genes (cag pathogenicity island) that codes a type IV secretion system that injects the CagA protein into gastric epithelial cells and is also associated with increased secretion of interleukin-8, a strong proinflammatory chemokine.14 It has been postulated that CagA evokes host systemic immune responses, producing autoantibodies that cross-react with host platelet surface antigens promoting platelet aggregation via immune complex formation with augmented platelet clearance rates resulting in thrombocytopenia.15
The few Brazilian studies that have evaluated the role of H. pylori infection in adult cITP patients were based on cohorts from southeastern Brazil.16 Although the prevalence of H. pylori infection is high (80%) in northeastern Brazil17 and infection is often intrafamilial with onset in early childhood.18 No study to our knowledge has evaluated the association between H. pylori and cITP in cohorts from this region.
ObjectiveThe purpose of the present study was therefore to evaluate the effect of H. pylori eradication therapy on platelet counts in a cohort of cITP patients from northeastern Brazil.
MethodsThis prospective, observational, study evaluated 28 patients with cITP selected from those who attended the Centro de Hematologia e Hemoterapia do Ceará, a referral center for cITP in Fortaleza, Ceara, Brazil. Patients, recruited through convenience sampling from August 2013 to August 2014, were followed up for one year.
cITP was diagnosed according to the guidelines of the American Society of Hematology.19 Inclusion criteria were i) platelet count <100×109/L for over six months, ii) normal or increased bone marrow megakaryocytes, and iii) no other thrombocytopenia-related conditions or factors. The exclusion criteria were i) platelet counts ≥100×109/L and ≤25×109/L, ii) treatment with corticosteroids, immunosuppressants or other specific drugs for cITP as well as splenectomy during the study period, iii) treatment with antimicrobial drugs, steroidal or non-steroidal anti-inflammatory agents or proton pump inhibitors in the 30 days preceding the study, iv) active gastrointestinal bleeding, v) pregnancy, vi) history of treatment for H. pylori infection, and vii) history of gastric surgery. cITP patients with platelet counts ≤25×109/L were excluded to reduce the confounding effect of concomitant cITP therapies during the study period.
Gastroendoscopy was performed at the university hospital Walter Cantideo, Fortaleza, Ceara, Brazil. All cITP patients were submitted to upper gastrointestinal endoscopy including biopsy of the gastric mucosa from the five sites recommended by the Houston-updated Sydney System for the Classification of Gastritis20 and to evaluate H. pylori status. Antral fragments from biopsies were submitted to the urease test.17 The biopsies were stained with hematoxylin and eosin and with Giemsa stains to evaluate the presence of H. pylori. Patients were considered H. pylori-negative when their results were negative in both histology and the rapid urease test.
Only H. pylori-positive patients were given eradication therapy (standard triple therapy: 20mg omeprazole and 500mg clarithromycin once a day and 1000mg amoxicillin twice a day, for one week). Patients with unsuccessful eradication were retreated with 20mg omeprazole and 500mg levofloxacin once a day and 1000mg amoxicillin twice a day, for one week.21
Eradication status was assessed using the 13C-urea breath test at least one month after the end of therapy.21 The sensitivity and specificity of the H. pylori detection test are 90% and 95%, respectively. Briefly, after a fasting period of six hours, the patient provided an initial breath sample, followed by the ingestion of 100mL orange juice containing 75mg 13C-urea. Thirty minutes later a second breath sample was collected. The samples were submitted to infrared spectrometry (IRIS, Wagner Analysen Technik GmbH, Bremen, Germany). The breath samples were considered positive if the delta-over-baseline value (DOB) was greater than 4%.22
To determine the platelet count, blood samples were collected at baseline and at 3, 6 and 12 months. Six months after therapy, the effect of the eradication therapy on platelet recovery was evaluated. Platelets were counted using an automated electronic counter (XT-1800i Automated Hematology Analyzer, Sysmex Corporation, Kobe, Japan).
Complete response to H. pylori eradication was defined as a final platelet count of >100×109/L with an increase of at least 30×109/L in comparison to baseline. Partial response was defined as an increase of at least 30×109/L compared to baseline.11,12,16 Patients displaying complete or partial response were considered ‘responders’. The remainder were considered ‘non-responders’.
Statistical analysisGroup differences were analyzed with the Statistical Package for the Social Sciences (SPSS) software for Windows, v. 17.0 (SPSS Inc., Chicago, Illinois). Pearson's chi-square test and Fisher's exact test were used for categorical variables, while Student's t-test was used for continuous variables. The results are expressed as means±standard deviation (SD). The level of statistical significance was set at 5% (p-value <0.05).
EthicsThe study protocol was approved on 10 July 2013 by the Research Ethics Committee of the Universidade Federal do Ceará (UFC), Brazil and filed under number 055.05.11. All participants gave their informed written consent. The study was conducted in accordance with the Helsinki declaration as revised in 2008.
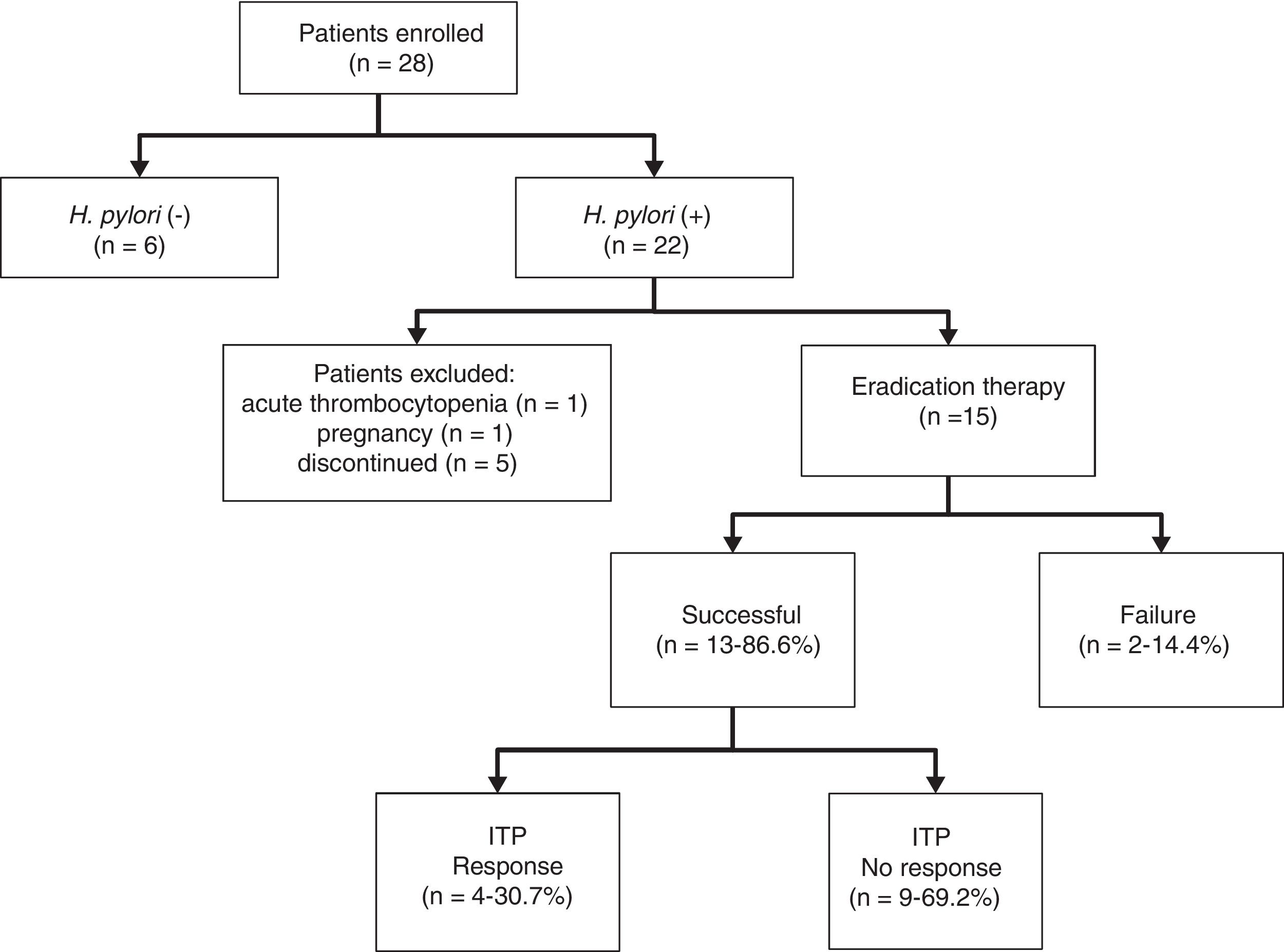
ResultsThe cohort consisted of 28 cITP patients, of whom 22 (78.5%) were H. pylori-positive. Five dropped out, one became pregnant, and one had a platelet count <25×109/L during the study period. The seven H. pylori-positive patients who were excluded did not differ significantly from the remaining 15 with regard to gender (male=2 and female=5 vs. male=1 and female=14; p-value=0.38 – chi-square), mean age (40.2±19.2 years vs. 47.4±12.6 years; p-value=0.70 – Student's t-test), baseline platelet count (73.45±25.00×109/L vs. 72.93±21.62×109/L; p-value=0.977 – Student's t-test) or cITP duration (10.8±3.6 years vs. 7.36±7.07 years; p-value=0.524 – Student's t-test). Figure 1 is a flowchart of the study from recruitment to outcomes.
The H. pylori-negative patients (n=6) were similar to infected patients (n=15) with regard to gender (female=5; p-value=0.115 – chi-square), mean age (25.6±15.6 years; p-value=0.411 – Student's t test), cITP duration (5.16±3.92 years; p-value=0.52 – Student's t-test), and baseline platelet count (70.40±20.08×109/L; p-value=0.96 – Student's t-test).
Six (40%) of the 15 H. pylori-positive patients had never been treated for cITP, five (33%) had a history of treatment with prednisone only and four (26%) had undergone splenectomy at least two years before the beginning of this study (two also received prednisone and two received prednisone plus immunosuppressants). Three (50%) of the H. pylori-negative patients had never been treated for cITP and three (50%) had a history of treatment with prednisone only. Note that no cITP patient in this study used immunosuppressants or corticosteroids for at least two months before the study. At endoscopy, antral gastritis was observed in five patients, corporal gastritis in three, pangastritis in two, and a duodenal ulcer in one. The remaining four patients displayed no histological abnormalities. H. pylori-negative patients had antral gastritis (n=3) or no abnormalities (n=3).
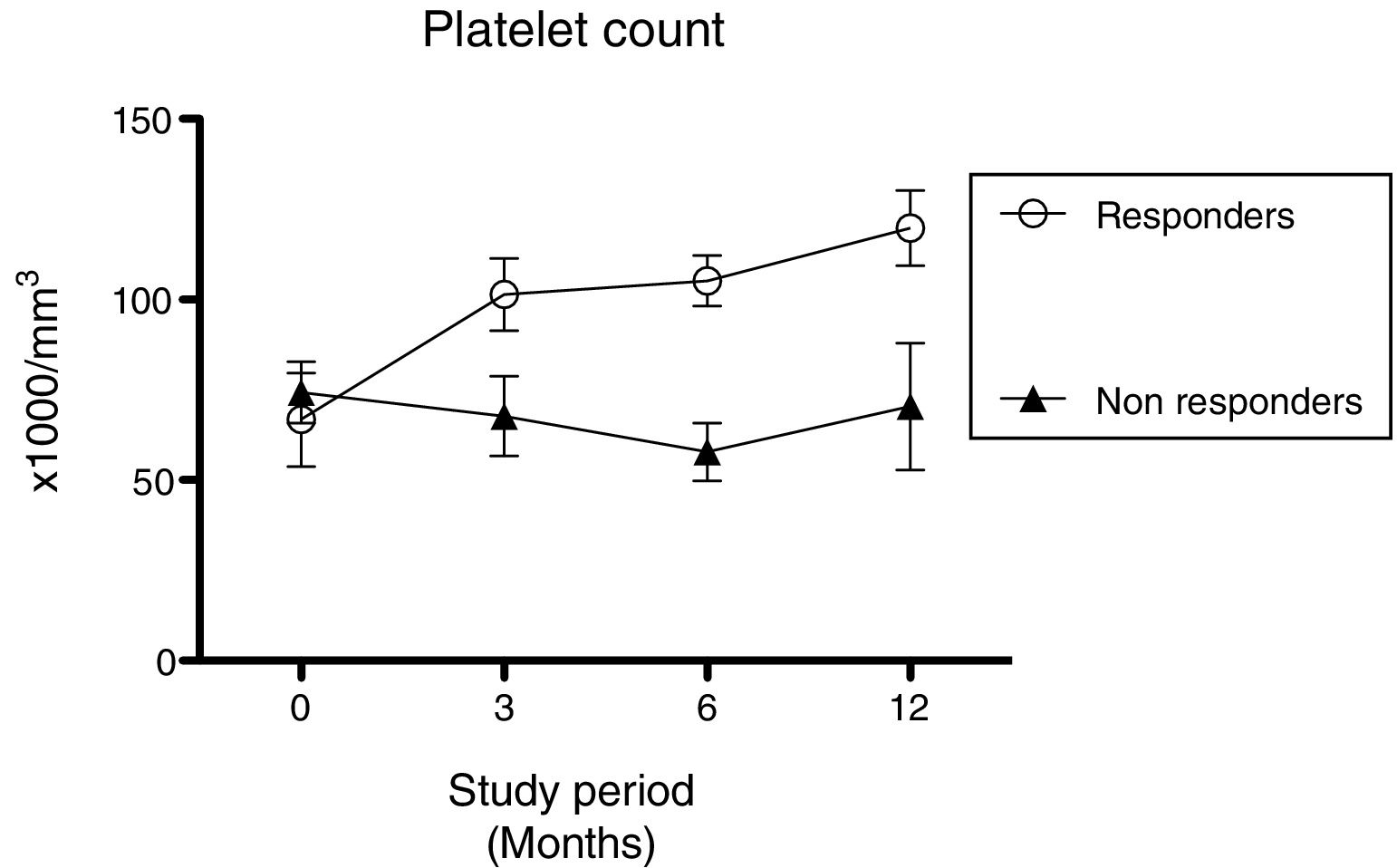
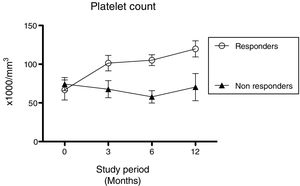
Eradication was successful in 13 (86.6%) of the 15 patients treated for H. pylori. Eradication failed in the remainder (n=2; 13.4%) even after retreatment. Six months after treatment, four patients (30.7%) displayed increased platelet counts. Half of these (n=2) were classified as complete responders and half as partial responders. Platelet counts were 112.8±24×109/L for responders and 57.7±24.0×109/L for non-responders at six months, and 119.8±20.7×109/L for responders and 70.9±49.9×109/L for non-responders at 12 months (Figure 2).
One of the four responders with increased platelet counts after six months had been submitted to splenectomy seven years prior to follow-up, two had a history of corticosteroid treatment, and one had never used medications. Age was <50 years for one patient (25%) and >50 years for three (75%).
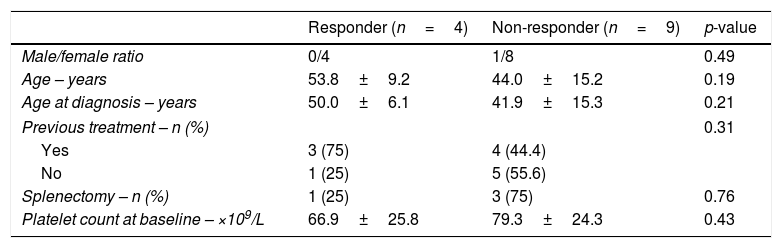
Responders and non-responders did not differ significantly with regard to gender, age at diagnosis, mean baseline platelet count, mean disease duration, history of immunosuppressive and corticosteroid therapy, or history of splenectomy (Table 1).
Clinical characteristics of patients with chronic idiopathic thrombocytopenic purpura (cITP) that successfully eradicated H. pylori.
| Responder (n=4) | Non-responder (n=9) | p-value | |
|---|---|---|---|
| Male/female ratio | 0/4 | 1/8 | 0.49 |
| Age – years | 53.8±9.2 | 44.0±15.2 | 0.19 |
| Age at diagnosis – years | 50.0±6.1 | 41.9±15.3 | 0.21 |
| Previous treatment – n (%) | 0.31 | ||
| Yes | 3 (75) | 4 (44.4) | |
| No | 1 (25) | 5 (55.6) | |
| Splenectomy – n (%) | 1 (25) | 3 (75) | 0.76 |
| Platelet count at baseline – ×109/L | 66.9±25.8 | 79.3±24.3 | 0.43 |
Data are presented as means±(standard deviation) or number and %. The p-values were calculated using the Statistical Package for the Social Sciences (SPSS) for Windows (SPSS Inc., Chicago, Illinois). Pearson's chi-square test and Fisher's exact test were used for gender, previous treatment and splenectomy variables, while Student's t-test was used for age, age at diagnosis and platelet count at baseline.
Fourteen (93%) of the 15 patients submitted to eradication therapy were followed up for 12 months. Persistent increases in platelet counts were observed in the four patients with initial responses (including one case in which splenectomy had been ineffective). The platelet count of non-responders remained low, and two received corticotherapy. No remission of cITP was observed at six months in two patients who were refractory to eradication; one presented hemorrhagic symptoms that were subsequently treated with prednisone. The platelet count of the six H. pylori-negative patients (baseline 72.1±19.6×109/L vs. 85.0±17.0×109/L at six months follow-up; p-value=0.134) remained practically unchanged during the 12-month follow-up, except for one patient who displayed a response at six months (103.0×109/L), which reverted to 89.3×109/L at twelve months. H. pylori-negative patients reported no bleeding or need for treatment, splenectomy or specific medications during the follow-up.
DiscussionThe association between H. pylori infection and cITP is based on the observation of improvements in platelet count following the eradication of H. pylori.8–11 However, the prevalence of H. pylori infection among cITP patients and the frequency of response in platelet count following eradication therapy vary greatly between geographical regions.23
Some reports indicate an increased prevalence of H. pylori infection among patients with cITP compared to the general population.9,11 In this study, the prevalence of H. pylori in cITP patients was 75.6%, similar to the findings of an earlier study by our group evaluating the prevalence of H. pylori among dyspeptic adults (80%) in Fortaleza, northeastern Brazil17 and the findings of studies from the southeast of Brazil,16 Italy7 and Japan.24
In the present study, the H. pylori eradication rate was 86.6% (13/15) using standard triple therapy. This is compatible with several other studies reporting eradication rates above 70%, with the exception of a single study from Japan (42.9%).23
Clinical response was positive in 30.7% of H. pylori-positive patients after eradication therapy. Similar post-eradication remission rates were reported from southeastern Brazil (17/59; 28.8%),16 a multicenter study conducted in Italy and England (17/52; 33.0%)25 and a study from Japan (6/22; 28%).26 Lower rates were reported in studies from Iran (3/41; 7.3%),27 Spain (3/23; 13%)12 and the United States (1/14; 7.1%),11 and higher rates were observed in studies in Colombia (21/26; 80.1%),11 Japan (10/15; 67%)9 and Korea (39/42; 92.9%).10 It is important to mention that higher rates were also observed in a study conducted in children and adolescent patients from the southeast of Brazil (6/10; 60%).28
Differences in clinical findings between responders and non-responders submitted to H. pylori eradication therapy have been the object of much study, but results have been variable. It has been suggested that platelet recovery in response to H. pylori eradication may be influenced by the duration of cITP,24 age at onset, baseline platelet count and history of prednisone therapy.25 In the present study, no association was found between background characteristics and platelet recovery rates, similar to other reports.8,26,29
Nearly all (93%) of the patients submitted to H. pylori eradication therapy were followed up for 12 months. At this time, platelet recovery was sustained in all four patients with initial response, even in a case of unsuccessful splenectomy. In contrast, platelet counts did not improve in patients with successful eradication but poor initial response, nor in patients with unsuccessful eradication. Generally, H. pylori-negative patients displayed no change in platelet count between baseline and six months, except for one patient with a slight improvement, who reverted at 12 months. Studies following cITP patients for longer periods found that in most cITP patients responding to H. pylori eradication therapy, improvement was sustained without idiopathic thrombocytopenic purpura treatment for more than 12 months.8,30 Thus, our results are in agreement with other studies and raise the possibility that treatment for cITP may be discontinued without any negative impact in this subgroup of patients.
The mechanism linking H. pylori infection to cITP is not fully understood, but some attribute it to the virulence factor of CagA. Infection with CagA-positive H. pylori strains conceivably induces the formation of platelet autoantibodies by chronic immune stimulation or the molecular mimicry of CagA to platelet antigens.15 Furthermore, clinical studies reinforce a correlation of H. pylori-specific CagA with clinical idiopathic thrombocytopenic purpura. It has been demonstrated that successful H. pylori eradication therapy in cITP patients improves the total circulating platelet count and decreases anti-CagA antibodies.24
This is the first study evaluating the effect of H. pylori eradication therapy on platelet count in patients with cITP from northeastern Brazil. The study was limited by the small sample size; however it is important to mention that cITP is not a common disease and it is difficult for a single center study to enroll a large number of patients. Another limitation is the short follow-up period. Further investigations are necessary to clarify the role of H. pylori infection in cITP.
ConclusionIn conclusion, cITP patients were found to have relatively low platelet recovery rates (31.4%) in response to H. pylori eradication therapy in this study, but the effect remained during one year of follow-up. These results suggest that treating H. pylori infection might be worthwhile in a subset of cITP patients. Further studies are required to understand the mechanism underlying the response to eradication therapy.
Financial supportFundação Cearense de Apoio à Pesquisa (FUNCAP), case filed under number 135067154.
Conflicts of interestThe authors declare no conflicts of interest.