Hematology Specialist Association 18. National Congress
Mais dadosDrug-induced leukemia is a recognized adverse drug reaction associated with specific classes of medications. This study aims to quantify the frequency of leukemia-related adverse drug reactions and identify the medications most implicated as potential causes in the FDA Adverse Event Reporting System (FAERS).
MethodsLeukemia-related adverse events were analyzed from the FAERS database spanning the period from 1969 to June 30, 2024. The search terms included various leukemia subtypes, such as “acute myeloid leukemia” (AML), “acute lymphoblastic leukemia” (ALL), “chronic myeloid leukemia” (CML), “chronic eosinophilic leukemia” (CEL), “chronic myelomonocytic leukemia” (CMML), “chronic lymphocytic leukemia” (CLL), and “hairy cell leukemia” (HCL). Cases were categorized by generic drug names, and the number of FAERS reports associating specific drugs with leukemia as an adverse event was determined. Descriptive statistics were applied to analyze the frequency, distribution, and outcomes of the reported cases.
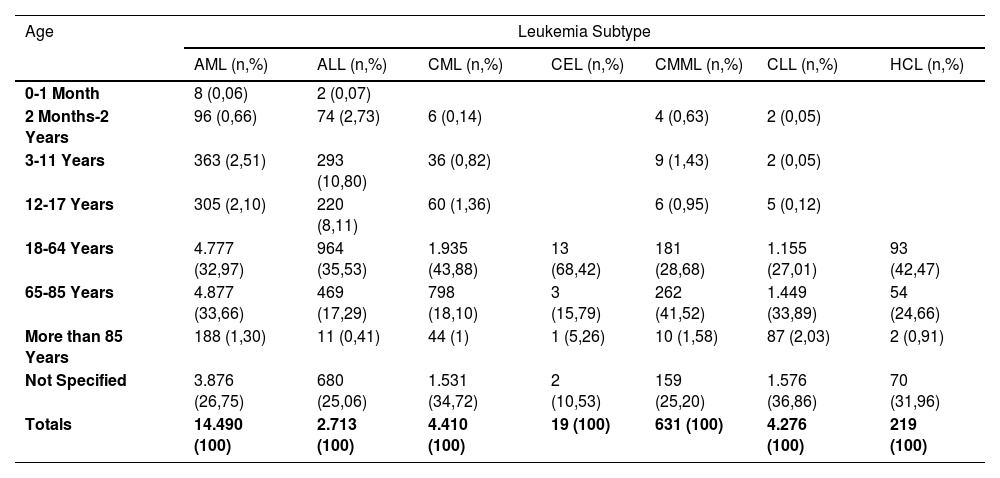
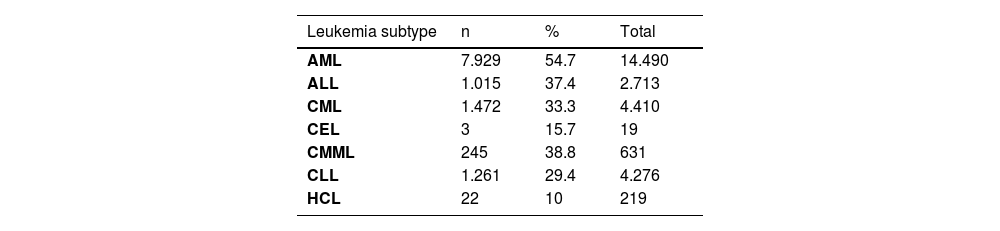
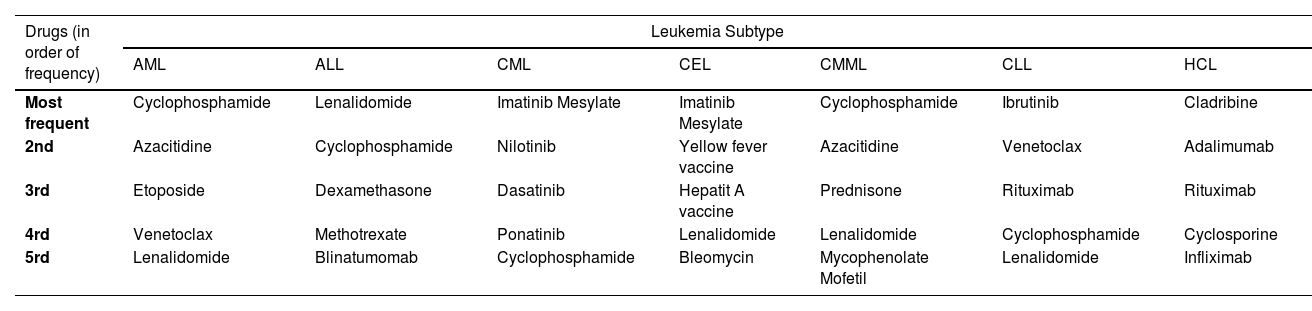
ResultsBetween 1969 and June 30, 2024, FAERS recorded a total of 29,153,222 adverse events, of which 26,758 (0.0009%) were linked to leukemia. Of these cases, 11,947 (31.9%) resulted in death. AML was the most frequently reported leukemia subtype, accounting for 14,490 cases, followed by CML with 4410 cases and CLL with 4,276 cases. The age distribution indicated that AML was predominantly reported among adults, with 4,777 cases (32.97%) occurring in individuals aged 18-64 years and 4,877 cases (33.66%) in those aged 65-85 years. Notably, the medications most frequently associated with leukemia were those primarily used in its treatment, including cyclophosphamide, lenalidomide, and imatinib mesylate.
ConclusionMedications commonly employed in the treatment of leukemia were frequently reported in FAERS due to concerns regarding their ineffectiveness rather than their expected therapeutic benefits. This highlights the critical need for continuous pharmacovigilance to ensure the efficacy and safety of these therapeutic agents.