The guidelines project is a joint initiative of the Associação Médica Brasileira and the Conselho Federal de Medicina. It aims to bring together information in the scientific literature to standardize conduct in order to help decision-making during treatment. The data contained in the following articles were prepared by and are recommended by the Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular (ABHH). Even so, all possible medical approaches should be evaluated by the physician responsible for treatment depending on patient's characteristics and clinical status.
Description of the method used to gather evidence.
These guidelines are the result of a systematic review centered on the Evidence-Based Medicine movement, where clinical experience is integrated with the ability to critically analyze and rationally apply scientific information, thereby improving the quality of medical care.
Questions are structured using the Patient/Problem, Intervention, Comparison and Outcome (PICO) system, allowing the identification of keywords which were the basis of evidence-based search strategies in the major scientific databases [Medline (via PubMed), EMBASE, Central (Cochrane), Lilacs (via BVS)] and manual searches.
Degree of recommendation and level of evidence
A: Major experimental and observational studies
B: Minor experimental and observational studies
C: Case reports (non-controlled studies)
D: Opinion without critical evaluation based on consensus, physiological studies or animal models
These guidelines are subdivided into five articles:
Part 1: Laboratory workup to confirm diagnosis
Part 2: Classification systems
Part 3: Treatment of low-risk patients without the 5q deletion
Part 4: Treatment of low-risk patients with the 5q deletion
Part 5: Treatment of high-risk disease
Part 1: Myelodysplastic Syndromes – Laboratory workup to confirm diagnosisObjective
This article presents the guidelines on the examinations that are needed to confirm the diagnosis of myelodysplastic syndromes.
PICO system
Using the PICO system, the P corresponds to patients with suspected myelodysplastic syndrome, I to the indication of examinations, and O to the outcomes (diagnosis).
Thus, 38 studies were found and selected to answer the clinical question (Appendix I).
What examinations are needed to confirm the diagnosis of myelodysplastic syndromes?
IntroductionMyelodysplastic syndromes (MDS) are a heterogeneous group of hematopoietic stem cell (HSC) clonal diseases resulting from a sequence of acquired genetic alterations with the formation of an anomalous and genetically unstable clone or clones, with potential to evolve to acute myeloid leukemia.
So far, there is no single reliable biological or genetic marker for diagnosis. Dysplastic alterations of peripheral blood (PB) and bone marrow (BM) are still fundamental for the diagnosis and classification of this group of diseases.
The detection of increased blasts facilitates diagnosis of the most advanced phases of the disease; however, in the early phases with minor morphological abnormalities, diagnosis is mainly based on the exclusion of other nonclonal cytopenias and diseases.
These clonal disorders may occur at any age, but they are more common in adults, with exponential increases after the 5th decade with a mean age at diagnosis of 70 years. Most patients acquire de novo clonal anomalies, with some acquiring somatic mutations after exposure to genotoxic agents, such as chemotherapy or radiotherapy for other neoplasms. Moreover, aging-associated inflammation (inflammaging) contributes to genetic instability and MDS predisposition.
The complete blood count with reticulocyte count and cytomorphological analysis are the vital first steps in the diagnosis of MDS. Isolated or combined, persistent for at least six months and unexplained cytopenias are common. Anemia, the most common cytopenia, is usually macrocytic, associated with a significant reduction in the reticulocyte count. All other causes of cytopenias/dysplasias should be excluded, as well as other clonal diseases and congenital abnormalities. Subsequently, a morphological analysis of BM cytological smears and an investigation of ring sideroblasts by Perls staining are performed. BM biopsy is complementary as it allows a better evaluation of the morphology and distribution of megakaryocytes, topography of other lineages, search for lymphoid aggregates and assessment of the degree of fibrosis by a standardized staining procedure. This is followed by classical and molecular cytogenetic analysis and immunophenotyping1(D).
Extraction of the ResultsMorphological diagnosisThe main criteria in the morphological evaluation is the observation of dyspoiesis or cellular atypia found in at least 10% of the cells of the lineage under consideration2(D),3(B). The main finding in cytology is a loss of cell uniformity compared to normal cells. Loss of architectural organization of the hematopoietic sector compared to its normal structure is found by histology. No single morphological finding is sufficient for the diagnosis for MDS.
Bone marrow cytologyAlthough cytopenia is seen in the PB, the BM is normocellular or hypercellular. Several features are observed in the erythroid lineage such as maturation delay, failures in hemoglobinization, asynchrony between nucleus and cytoplasmic maturation, megaloblastoid changes, anomalous mitoses, multinuclearity, nuclear chromatin bridges, nuclear budding and ring sideroblasts.
In the granulocytic lineage, one might observe: maturation delay translated by the accumulations of the phases of myeloblasts and myelocytes or even metamyelocytes with multiple isolated nuclei, asynchrony between nucleus and cytoplasmic maturation, giant metamyelocytes, degranulation (hypogranularity and/or heterogeneous distribution within the cytoplasm), the pseudo-Pelger Hüet anomaly, nuclear anomalies (hyposegmentation), donut-shaped cells, and bizarre, atypical, aberrant immature forms. Auer rods or bodies may be found. The blasts count in the BM is important not only as a criterion for diagnosis and classification, but also as it has a strong impact on the prognosis. Type I, II and III myeloblasts may coexist in the same patient, in the same sample and, in some cases, at different times. The megakaryocytic lineage is the most characteristic marker of dyspoiesis when present. Hypersegmentation, dumbbell-shaped nuclei, large or small mononuclear megakaryocytes and micro-megakaryocytes can be observed4(D).
Bone marrow histologyCellularity, established as the percentage of parenchyma and adipocytes, should be checked by histology allowing the classification of BM as hypocellular, normocellular, or hypercellular. Interpretation should consider the normal range for age. Abnormal distribution of cellularity may occur. Ectopia is the abnormal location of cells: megakaryocytes and erythroblasts in a paratrabecular location, clusters of myeloblasts and/or promyelocytes containing >5 cells in the intertrabecular region of marrow (ALIPs - Abnormal Localization of Immature Precursors). An evaluation of the reticulin network is important, as it has shown prognostic significance. Lymphoid aggregates are present in about 20% of cases5–7(D).
The hyperfibrotic subtype of MDS presents significant degrees of medullary fibrosis; European Consensus Grades II and III occur in 10% of cases and have a more aggressive clinical course often associated with excess blasts8(D),9,10(B). The diagnosis is always based on histology. As BM fibrosis is not exclusive to MDS but occurs in other myeloid diseases, an accurate clinical evaluation is recommended as well as a search for evidence of dyspoiesis in the PB; a biopsy imprint may help define this criterion as there is often insufficient material for cytological evaluations due to the fibrosis11(D). Focal fibrosis was observed in 17% of cases of a large cohort with only 5% having a diffuse and compact pattern12(B).
Hypoplastic MDS is characterized by cellularity ≤30% in over 60-year-old patients; it is more common after chemotherapy or radiotherapy. This subtype corresponds to 10–15% of the cases and usually demands special attention in the differential diagnosis with severe aplastic anemia, which may be decisive for correct conditioning before BM transplantation13(D),14–16(B). The distinction between hypoplastic MDS and aplastic anemia is challenging in terms of morphology.
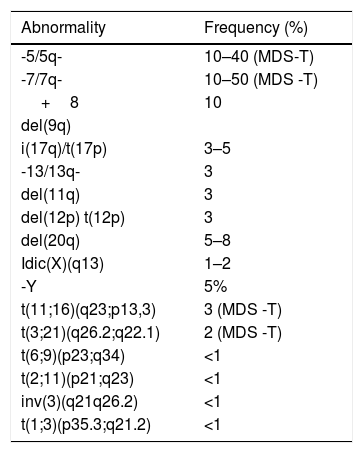
Conventional and Molecular CytogeneticsCytogenetic analysis plays an important role in determining clonality in patients with suspected MDS. Cytogenetic abnormalities are observed in 50–60% of patients, with the most common anomalies being del(5q), monosomy or deletion of 7q, trisomy 8 and del(20q). The diagnosis of MDS can be made in the absence of morphological dysplasia when there are recurrent cytopenias and cytogenetic abnormalities as described in the WHO 2008 classification (Table 1)17(D). However, nullisomy Y (-Y), 8 trisomy, and del(20q) are cytogenetic abnormalities that, in the absence of dysplasia, are not considered definitive evidence of MDS as they can be seen in other myeloproliferative diseases4,17(D).
Recurrent cytogenetic abnormalities and their frequency in myelodysplastic syndromes at diagnosis17(D)
| Abnormality | Frequency (%) |
|---|---|
| -5/5q- | 10–40 (MDS-T) |
| -7/7q- | 10–50 (MDS -T) |
| +8 | 10 |
| del(9q) | |
| i(17q)/t(17p) | 3–5 |
| -13/13q- | 3 |
| del(11q) | 3 |
| del(12p) t(12p) | 3 |
| del(20q) | 5–8 |
| Idic(X)(q13) | 1–2 |
| -Y | 5% |
| t(11;16)(q23;p13,3) | 3 (MDS -T) |
| t(3;21)(q26.2;q22.1) | 2 (MDS -T) |
| t(6;9)(p23;q34) | <1 |
| t(2;11)(p21;q23) | <1 |
| inv(3)(q21q26.2) | <1 |
| t(1;3)(p35.3;q21.2) | <1 |
MDS-T: Therapy-related myelodysplastic syndrome
*Nullisomy Y (-Y), 8 trisomy and del(20q) are cytogenetic abnormalities that are not considered definitive evidence of MDS
In the case of repeated failure of conventional cytogenetic investigations (G-band karyotyping) due to the absence or poor quality of metaphases, fluorescence in situ hybridization (FISH) may complement the cytogenetic analysis.
BM samples from 48 MDS patients (43 low risk) were evaluated by karyotyping and FISH using probes for chromosomes 5, 7, 8, 11, 13 and 20. Eighteen of the 48 samples (37.5%) had an abnormal clone identified by metaphase chromosomal analysis, while 17 of 48 (35.5%) had an abnormal clone detected by FISH analysis. Normal karyotypes were detected in 30 of the 48 patients (62.5%) with 29 of these 30 having a normal FISH result. The sensitivities of the two methodologies (FISH and karyotyping) were very similar and so FISH may be a good tool to study BM aspirate when cytogenetic analysis is not available18,19(B).
In another study, 433 BM samples from patients with suspected MDS or AML were submitted to karyotyping and FISH for -5/5q-, -7/7q-, +8 and 20q-. Abnormal karyotypes were identified in 114 cases (26.3%) and abnormal FISH results of at least one locus were found in 94 cases (21.7%). Abnormal results by FISH or karyotyping were detected in 136/433 samples (31.4%). Of the 136 cases with any abnormal finding, 22 (16.2%) were identified only by FISH. The two methods had concordance in 96.5%, 96.5%, 93.9% and 93% of the cases of abnormalities of chromosomes 5, 7, 8 and 20, respectively20(B).
Molecular geneticsArray-based karyotyping is a novel technique that allows the detection of chromosomal abnormalities not observed by conventional cytogenetics, chiefly with the use of single nucleotide polymorphism arrays (SNP-arrays)21(D),22(B). Somatic mutations are observed in 80–90% of MDS patients with the most commonly affected genes being SF3B1, TET2, SRSF2, ASXL1, DNMT3A, RUNX1, U2AF1, TP53 and EZH2. However, these mutations, in addition to being observed in other neoplasms, in particular myeloid neoplasms, may also occur in apparently healthy elderly patients as the so-called ‘clonal hematopoiesis of indeterminate potential’ (CHIP)23(D). Although some patients may progress to MDS, the natural course of these cases has not yet been elucidated and the presence of these somatic mutations associated with MDS was not included as a diagnostic criterion in the WHO 2016 classification4(D). As with cytogenetic abnormalities, the type and number of mutations are associated with the evolution/progression of MDS. Mutations of TP53 are associated with the most aggressive type of disease and, in these cases, evaluation is indicated, particularly in respect to MDS with isolated del(5q), which determines a less favorable prognosis and seems to predict an unfavorable response to lenalidomide24(B).
The SF3B1 mutation was described in 30% of MDS cases, and in 80% of MDS with ring sideroblasts, that is, this mutation is predictive of ring sideroblasts25(B). According to the WHO 2016 classification, cases of suspected MDS who have between 5 and 15% of ring sideroblasts should be investigated for this mutation with the diagnosis being conclusive when it is present.
Generally, patients with MDS with ring sideroblasts and SF3B1 mutations (most frequently in exon 15 - K700E) are elderly with low-risk international prognostic score (IPSS) who present better overall survival, greater event-free survival and favorable karyotypes26(B). Patients with MDS with ring sideroblasts and no SF3B1 mutations in general present TP53 mutations and worse outcomes.
Recommendations
Conventional cytogenetic analysis (G-band karyotyping) is an integral part of the evaluation of patients with suspected MDS. The results may not be informative when an insufficient number of cell metaphases (<20 metaphases) is obtained due to hypocellularity or in the absence of cell growth.
FISH studies, in particular chromosomes 5, 7, 8 and 20, are useful in patients for whom several cultures of bone marrow were performed without obtaining metaphases. This study may also help in the elucidation of complex chromosomal abnormalities. FISH analysis should not replace conventional karyotyping as an analysis of the whole set of chromosomes is essential.
Array-based karyotyping is complementary to metaphasic karyotyping and can be added to diagnostic tests however it is expensive and not widely available in Brazil. In addition, the detection of somatic mutations, even though observed in 80–90% of MDS cases, does not yet have a clear role in the diagnostic evaluation of unexplained cytopenias. Mutation screening of TP53 is indicated particularly in MDS patients with del(5q) and screening for SF3B1 mutations in patients with between 5–14% of ring sideroblasts in the BM.
Immunophenotyping by flow cytometry (FCM) allows an analysis of the antigen expression related to lineage and degree of maturation in normal and abnormal hematopoiesis27(B). The expression of this antigen is tightly controlled by genetic and epigenetic mechanisms, leading to a predictable normal pattern at different stages of maturation28(B). In MDS, there is abnormal granulocytic, monocytic, and erythroid differentiation resulting in deviations from the normal pattern. There may be an increase or decrease in antigen expression, asynchronous expression, or aberrant cross-lineage antigen expression. This can be detected by FCM. Thus, this technique is an important ancillary tool in the diagnosis of MDS cases where few morphological abnormalities are found in BM cytology and histology and the karyotype is normal28–31,33(B),32,34(D).
In the last 15 years, numerous papers have been published about this subject, and international study groups, such as the European LeukemiaNet, have standardized this analysis for 4- and 8-color panels32(D),33(B). In contrast to morphology, that is susceptible to subjective evaluation, FCM analysis is an objective method if well standardized. Furthermore, in cytological analysis it is recommended to count 500 cells, while in FCM at least 100,000 cells (and >250 CD34+ cells) should be analyzed for the diagnosis of MDS. Immunophenotyping of BM cells is able to detect numerous maturation abnormalities in the myelomonocytic and erythroblastic lineages as well as analysis of myeloid and lymphoid progenitors34(B).
Increases of myeloid CD34+ cells often presenting aberrant and cross-lineage antigen expressions may be found, especially in refractory anemia with excess of blasts (RAEB)29–31,34–38(B). Moreover, B-cell precursor counts (hematogones type I) are lower compared to the normal number for the age of the patient33,39(B).
No fixed antibody panels or combinations have been recommended32(D),34(B). The analysis performed must always be compared to the findings of age-matched normal BM (BM donors, orthopedic surgery patients, etc.)32(D),29,31,33–35,39(B). FCM abnormalities found should be interpreted in the context of the clinical findings and other laboratory results, such as BM cytology and cytogenetics. There is no single pathognomonic abnormality for MDS. International recommendations establish that all abnormalities should be reported and the diagnosis of MDS is suspected if at least two aberrations are found.
Several efforts have been made to establish diagnostic scores but there is much controversy in the literature concerning this subject and none of the described scores is universally accepted21(D),33(B). The most commonly used in the clinical practice is the Flow Cytometry-based Scoring System (FCSS)40(B) based on several parameters of maturation abnormalities of the myelomonocytic series and increases and phenotypic aberrancies of myeloid progenitors. A simpler score (Ogata score) is based on four parameters: CD34+ myeloblast-related and B-progenitor-related cluster sizes, CD45 expression of myeloblasts and granulocyte side scatter using the values obtained for these parameters in the lymphocyte gate as an internal control. This score has a sensitivity of 70% and a specificity of 93% for the diagnosis of MDS41(B).
In earlier studies there was a concern to compare the findings of FCM with those of BM cytology and cytogenetics28,30,36(B). However, in more recent papers, as FCM has been well standardized, the emphasis is placed on a comparison with normal age-matched BM and how much the variables obtained in MDS differ from normal findings. Thus, it is important that each laboratory working in this field has its own local normal control values.
Nowadays, immunophenotyping in MDS has been well standardized, and based on the knowledge of the normal antigen expression of the several hematopoietic precursors in BM. Usually one or two standard deviations from the normal mean fluorescence intensity is used to say that there is an abnormal expression of an antigen. Also, it is more important to have local normal values of CD34+ cells rather than to use fixed normal values described in the literature, as these values may change with the fluorochromes conjugated to the monoclonal antibodies, the gating strategy used, quality controls of each laboratory as well as patients’ age31(B),32(D). This technique demands good training of the operator but it is easily feasible in a routine laboratory and is useful to discriminate between reactive (non-clonal) peripheral cytopenias and low grade MDS with few BM cell atypias29,33,40,41(B).
FCM analysis of BM cells is also able to provide prognostic parameters, associated with survival, response to treatment and BM transplantation32,36,40(B),42(D). In a cohort of 101 Brazilian patients, the number of CD34+ myeloblast-related cells was an independent prognostic parameter compared to the WHO Classification-Based Prognostic Scoring System (WPSS) and Revised IPSS (IPSS-R), and had higher prognostic value than IPSS.
FCM is considered an ancillary technique in the diagnosis of MDS and should always be reported and interpreted together with the PB values, BM cytology and cytogenetics which remain the gold standard for diagnosis29(B). However, when the karyotype is normal, and cytology is inconclusive, FCM is a valuable technique to confirm the diagnosis4(D).
Recommendations
Immunophenotyping of BM hematopoietic precursors should be performed in patients with peripheral cytopenias stable for at least six months where BM morphology and cytogenetics do not give a clear-cut diagnosis. It is also useful to evaluate prognosis of the patient and to monitor response to treatment.
The authors declare no conflicts of interest.
1. Clinical question
What examinations are needed to confirm the diagnosis of myelodysplastic syndromes?
2. Structured question (PICO)
Patient Patients with MDS
Intervention Bone marrow aspiration with iron staining (Perls)
Bone marrow biopsy (hematoxylin-eosin and reticulin)
Bone marrow karyotype
Fluorescence in situ hybridization (FISH) panel (monosomy/deletion of chromosomes 5 and 7 and trisomy 8)
Bone marrow immunophenotyping by flow cytometry to investigate quantitative alterations and alterations of antigen expression in hematopoietic precursors of the bone marrow
Comparison None
Outcome Diagnosis, confirmation
3. Initial eligibility criteria for studies
- •
Components of PICO
- •
No time limit
- •
No limit of languages
- •
Full text availability
4. Search strategies
- •
#1: (Myelodysplastic Syndrome OR Myelodysplastic Syndromes OR Dysmyelopoietic Syndromes OR Dysmyelopoietic Syndrome OR Hematopoietic Myelodysplasia OR Hematopoietic Myelodysplasias)=22.751
- •
#2: (Myeloid Neoplasms OR Pancytopenia OR Anemia, Refractory OR Lymphoid Neoplasms OR Primary Myelofibrosis OR Bone Marrow=362.675
5. Selection of articles
Initially selected by the title, sequentially by the abstract, and finally by the full text, the latter being subjected to critical evaluation and extraction of outcomes related to the outcomes.
6. Critical evaluation and strength of evidence
The strength of the evidence of the studies was defined taking into account the study design and the corresponding risks of bias, the results of the analysis (magnitude and precision), relevance and applicability (Oxford/GRADE).43,44