In December 2019, the first cases of a severe acute respiratory syndrome caused by coronavirus 2 (SARS-CoV-2; COVID-19) were detected in Wuhan, China.1 SARS-coV-2 is a highly infectious virus which leads to heterogeneous clinical manifestations.1 Some individuals remain asymptomatic, while others, especially individuals with comorbidities, are more prone to develop severe manifestations of the disease.2 SARS-CoV-2 leads to extensive inflammation, endothelial damage, platelet activation and hypercoagulability, characterizing a prothrombotic state.2
Advanced age, diabetes, obesity and hypertension are associated with a higher risk of thromboembolic complications in COVID-19.2 However, little is known about potential interaction between COVID-19 and hereditary thrombophilia and its effect on thrombotic risk.3
Antithrombin (AT) is a physiological inhibitor of coagulation, mainly of thrombin, but also of factors Xa, IXa and others.4 AT deficiency is an autosomal dominant hereditary disorder, with variable penetrance and equal distribution between sexes.4 Patients with AT deficiency are at significantly increased risk of thromboembolism, especially venous (VTE).4 Of all hereditary thrombophilias, AT deficiency is the one with the highest risk of VTE.4
The aim of this article is to report a case of a young man with AT deficiency and prothrombin mutation G20210A who, 7 days after the onset of COVID-19, developed an episode of deep venous thrombosis (DVT) in the right leg.
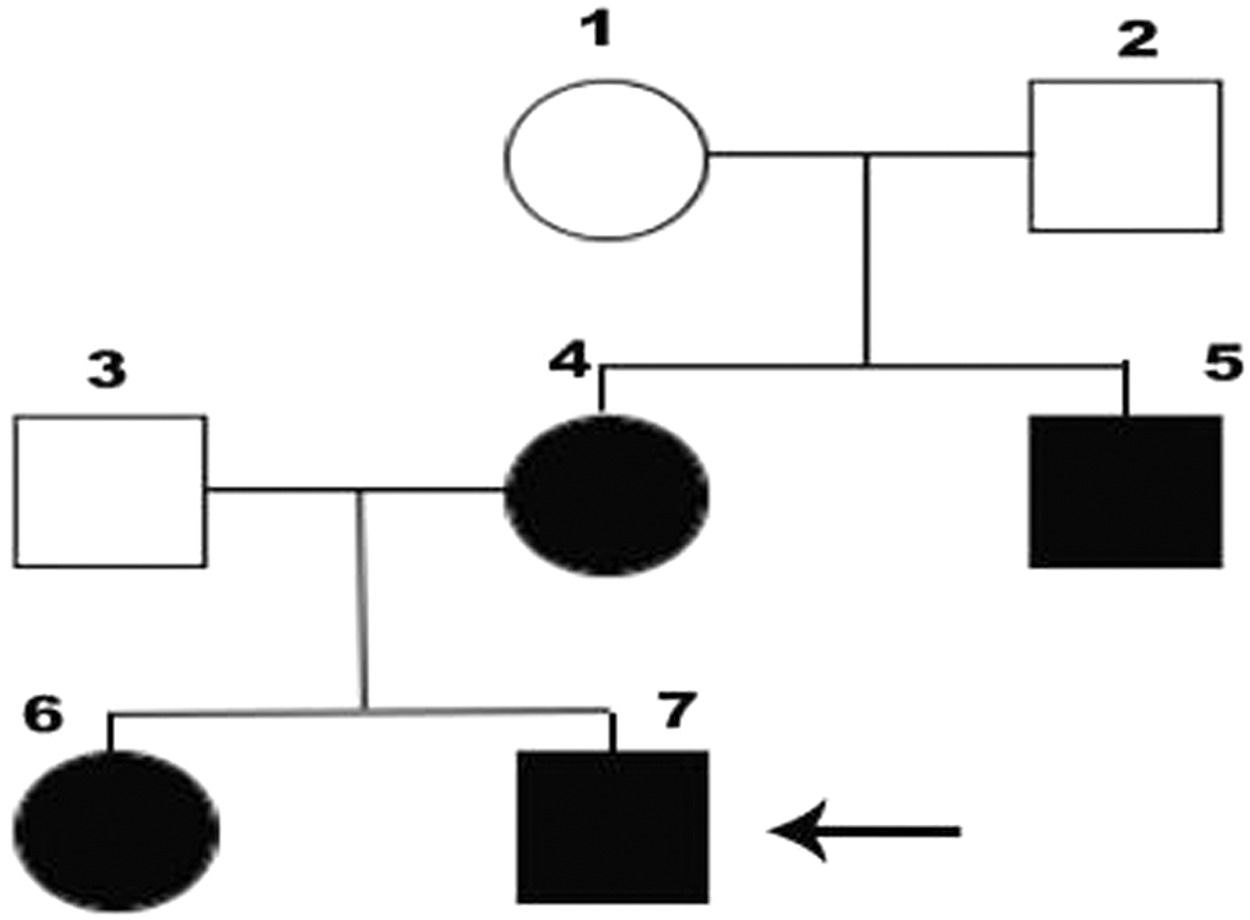
Case reportWe attended a 39-year-old male with known AT deficiency. He was diagnosed as part of a family screening for AT deficiency and had AT activity level was 58.6% (reference value, 79.0%-112.0%). He also carried heterozygosity for prothrombin mutation G20210A. The patient had no previous history of VTE or other comorbidities. His mother, maternal uncle and sister also have AT deficiency and had at least one episode of DVT before age 50 (Figure 1). They were under secondary prophylaxis with warfarin.
At consultation he had fever, nose running, anosmia and ageusia. Real-time polymerase chain reaction test for COVID-19 was positive and he was instructed to remain at home quarantine. On day 7 of infection, he noticed pain and swelling in the right lower limb. Fifteen days later, he returned to the medical service with respiratory symptoms, persistent pain and swelling in the right lower limb. He was then admitted, and a venous Duplex Scan confirmed an acute thrombus in the distal femoral and popliteal segment of the right leg. He was treated with enoxaparin, followed by warfarin to a target INR between 2 and 3. He remained hospitalized for four days and was then discharged, with no need of ventilatory support.
Black circles and squares represent individuals with AT deficiency. 7, index case; 4, mother; 5, aunt; 6, sister; 1, 2 and 3, status for AT deficiency unknown.
DiscussionWe presented a case of a 39-year-old man with AT deficiency and heterozygosity for the G20210A prothrombin mutation, who presented a DVT during the course of COVID-19. He had no other comorbidity, except for the hereditary thrombophilia. He had no previous thrombotic event and had never been hospitalized.
Due to the high incidence of VTE in patients hospitalized with COVID-19, thromboprophylaxis is recommended, unless there is a contraindication to it.5,6 The International Society on Thrombosis and Haemostasis5 and the Guidance on diagnosis, prevention and treatment of thromboembolic complications in COVID-19: a position paper of the Brazilian Society of Thrombosis and Hemostasis and the Thrombosis and Hemostasis Committee of the Brazilian Association of Hematology, Hemotherapy and Cellular Therapy6 recommends prophylactic anticoagulation with LMWH as early as possible for hospitalized patients with COVID-19. However, it is known that some patients with COVID-19 may develop VTE even before hospital admission.7 To assess the effectiveness of apixaban or aspirin (in comparison with placebo) in reducing major adverse cardiopulmonary outcomes among symptomatic, clinically stable outpatients with COVID-19, The ACTIV-4B Trial was initiated but did not demonstrate any reduction at the rate of a composite clinical outcome.8 Other studies, such as The Swiss OVID study, still in progress, aims to investigate whether prophylactic-dose enoxaparin improves survival and reduces hospitalizations in symptomatic ambulatory patients aged 50 or older diagnosed with COVID-19.9 Moreover, the ongoing PREVENT-HD randomized clinical trial (NCT 04508023) aims to analyze the effectiveness of rivaroxaban in reducing the risk of major venous and arterial thromboembolic events, all-cause hospitalization, and all-cause mortality, in comparison with placebo, in outpatients with COVID-19.10 Therefore, there is insufficient data to support the indication of broad thromboprophylaxis for outpatients with COVID-19.
The most common presentations of AT deficiency are DVT of the legs and arms and pulmonary thromboembolism (PE).4 The risk of occurrence of VTE increases from the age of 20 years onwards. Approximately 50% of affected patients will present at least one episode of VTE up to the age of 50. This was the case with all the family members of our patient, who developed VTE before the age of 50. Approximately 60% of the thromboembolic events in patients with AT deficiency occur as unprovoked and 40% as provoked.4 This was the case of this patient, with COVID-19 functioning as a trigger to VTE.
Only one study has investigated the occurrence of thrombosis in individuals with hereditary thrombophilias and COVID-19.3 In this study, most patients with severe AT deficiency did not develop thrombosis. Moreover, hereditary thrombophilia was not associated with more severe COVID-19. However, due to the small sample size and reduced number of events they could not conclude about the effects of potential interaction.3
Although there are ongoing trials on thromboprophylaxis in outpatients with COVID-19, in the view of the rarity of AT deficiency and its coexistence with other hereditary thrombophilia, it is highly unlikely that ongoing randomized clinical trials will provide evidence-based recommendations for these rare cases in the context of COVID-19.
In conclusion, we presented a case of a patient with AT deficiency and G20210A prothrombin mutation who developed, for the first time, a DVT during the course of COVID-19. Despite the presence of multiple thrombophilia and a strong family history for VTE, COVID-19 likely acted as a trigger for the development of the DVT in this patient. We, therefore, advice physicians to closely monitor individuals with known thrombophilia who develop COVID-19. A prompt diagnosis and treatment of VTE could avoid major complications.