Prophylaxis is the treatment of choice for patients with severe hemophilia. Low adherence may limit the effectiveness of the prophylactic regimen, thereby compromising outcomes.
ObjectiveThe objective of this study was to validate the Brazilian version of the VERITAS-Pro prophylaxis adherence scale, originally an American questionnaire that can be answered by the individual responsible for prophylaxis as well as by an observer.
MethodsThe scale has 24 questions divided into six subscales: Routine, Dosage, Plan, Remember, Skip and Communicate. Participants were recruited at a blood center in southeastern Brazil for validation and reliability analyses. Validation measures included the results obtained using analog visual scales of adherence, interval between medication dispensed by the treatment center pharmacy and the percentage of recommended doses administered and infusions registered in the patients’ logs.
ResultsThe study included 32 individuals responsible for prophylaxis and five observers. The internal consistency was very good for the VERITAS-Pro total score, excellent for the Remember, Skip and Communicate subscales, good for the Dosage subscale, and acceptable for the Routine and Plan subscales. Twelve participants answered the questionnaire on more than one occasion to evaluate reproducibility. The intraclass correlation coefficient was excellent. Regarding convergent validity, the VERITAS-Pro scores were moderately correlated with the global adherence scale and with infusion log records, but showed a weak correlation with pharmacy dispensation records.
ConclusionThe Brazilian version of VERITAS-Pro is a valid and reliable instrument, enabling the understanding of specific factors related to non-adherence and allowing targeted interventions for proper treatment.
Prophylaxis in hemophilia consists of regular infusions of clotting factor VIII or IX concentrate for a period longer than eight consecutive weeks in order to prevent bleeding.1,2 The benefits of prophylaxis include decreased frequency of bleeding episodes, decreased need for emergency room visits and hospitalizations, prevention of arthropathy, increased physical activity and school attendance, and improved academic performance.1,3–5 Prophylaxis reduces long-term morbidity, thus improving quality of life.6,7 The World Health Organization recommends prophylaxis as standard therapy for people with severe hemophilia.2,8
Non-adherence to the prophylactic regimen may limit the effectiveness of treatment with less prevention of bleeding.9 The lack of standardized methods to assess adherence to hemophilia prophylaxis limits the understanding of factors that facilitate or hinder the therapeutic program. Lack of awareness of adherence as a determining factor in health outcomes can lead to a waste of human and economic resources as well as underutilization of available medications.10,11
VERITAS-Pro is a questionnaire created in the United States, based on focus groups, to assess specific components of adherence as well as global adherence to the proposed prophylactic regimen.12 The objective of this study was to describe the psychometric properties of the Brazilian version of the VERITAS-Pro, demonstrating its usefulness as an instrument for clinical practice and research.
MethodsTranslation and adaptationVERITAS-Pro, originally developed in English, was translated into Brazilian Portuguese according to international translation and adaptation guidelines.13 Two independent translations to Brazilian Portuguese were prepared by native Brazilians. There were no relevant differences between the translations. The two resulting versions were combined, corrected by experts, and translated back into English by an American translator without knowledge of the original document. The Brazilian version has 24 questions divided into six subscales: Time, Dose, Plan, Remember, Skip, and Communicate, just as in the original questionnaire. The questions were written in a way that made it possible for both the individual responsible for the patient's prophylaxis and an observer to respond. The answers are presented as five-point Likert scales ranging from ‘never’ to ‘always’. An ‘always’ response reflects the best possible adherence for some items, and the worst for others. For each item, a numerical classification was assigned to the Likert scale, giving one point to the response representing the best adherence and five points to the worst adherence. Possible scores of each subscale range from 4 to 20 points, and the total score of the instrument, from 24 to 120 points, where 120 represents the worst adherence.
ParticipantsParticipants were recruited at the Hemocentro Regional de Juiz de Fora (HRJF) in southeastern Brazil. The individuals considered eligible were those responsible for the prophylaxis of patients with hemophilia with a severe phenotype, A or B, in a home infusion regimen for at least six months. Patients in on-demand treatment, those not qualified to receive home infusions, and those with inhibitors were excluded.
Participant recruitment and data collectionThe study was approved beforehand by the Research Ethics Committee of the Fundação Centro de Hematologia e Hemoterapia de Minas Gerais – HEMOMINAS. The participants were asked to respond to the questionnaire during their usual visits to the blood center. All signed a free and informed consent form, allowing access to their infusion logs, and received a guarantee of confidentiality of any individual information. Parents gave their consent to include data from patients under 18 years of age, and adolescent patients (12–17 years old) gave their agreement to participate. Sociodemographic and health data were collected from the patients, as well as sociodemographic data from those responsible for prophylaxis, in the case of under 18-year-old and incapacitated patients. Participants answered a questionnaire and a visual analog scale on global adherence to prophylaxis. For the test–retest evaluation, participants were asked to respond the questionnaire a second time on their next visit to the blood center. Data collection occurred between October 2015 and November 2016.
Other measures of adherence to prophylaxisAnalog scale of global adherence to prophylaxisAfter completing the VERITAS-Pro, participants were reminded of the details of the prescribed prophylaxis. An analog scale with values ranging from zero (never follow the prescription) to 10 (always follow the prescription) was then presented and the participants were asked to rate their adherence in the previous three months.
Records of medications dispensed by the blood center pharmacyThe number of doses dispensed was divided by the number of doses required to comply with the prescribed prophylaxis in the interval between dispensations. The analysis included dispensations that provided sufficient factor for the previous twelve weeks of treatment. If the individuals responsible returned later than the expected time for taking the required doses, it became evident that prophylactic infusions were skipped. However, if they returned at shorter than expected intervals, as it was not possible to determine by this method the reason for consumption greater than that prescribed, the highest possible adherence was considered 100%.
Percentage of recommended doses administeredThe percentage of doses administered on the correct days in relation to the doses prescribed in the three months prior to the application of the VERITAS-Pro was calculated from the infusion logs filled out by those responsible for the patient's prophylaxis. When clearly documented, doses to treat bleeding events were excluded. However, extra prophylactic doses infused prior to medical and dental procedures, or unusual physical activity, were included. Thus, the prophylaxis measured by this method could be greater than 100%, but for analytical statistics reasons, 100% was considered the maximum possible adherence value.
Statistical analysisReliability and validityInternal consistency was assessed using Cronbach's alpha. Since the subscales of the instrument contain only four items each, the following considerations were used: 0.8 characterized excellent internal consistency; 0.7, very good; 0.6, good; and >0.5, minimally acceptable.12 The intraclass correlation coefficient (ICC) was used to evaluate the VERITAS-Pro scores in the test–retest reliability analysis, which was considered excellent when >0.75.13 The Spearman correlation test was used to compare VERITAS-Pro results with the other adherence measures.14 Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS) v.20 software.
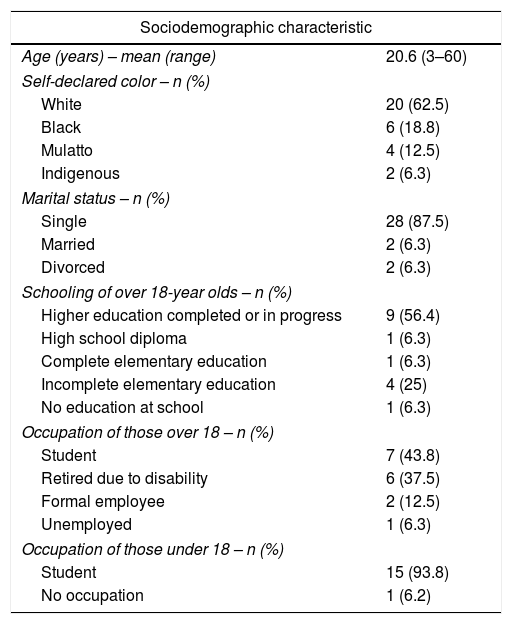
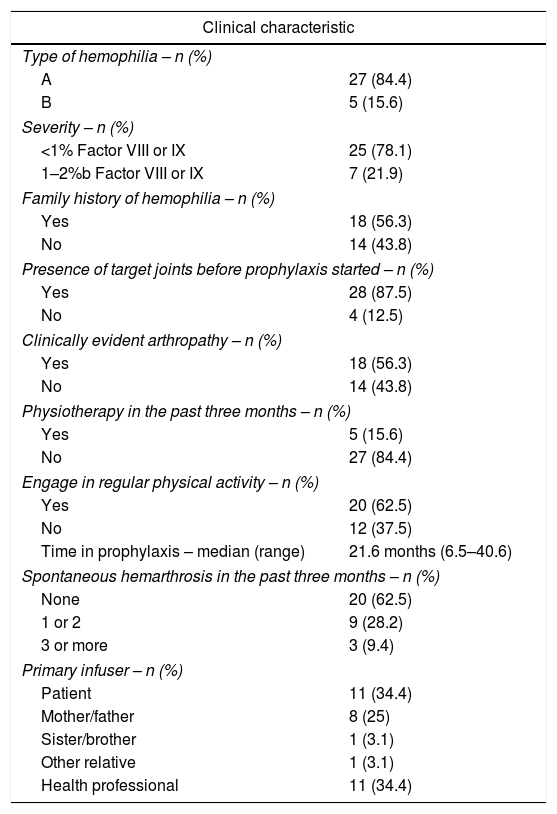
ResultsParticipantsAt the beginning of the study, in October 2015, there were 59 patients eligible for the prophylaxis regimen at the HRJF. Twenty-three patients (39%) had opted to remain on episodic treatment and one tested positive for an inhibitor (1.7%). Of the 36 patients who adhered to the prophylactic treatment, one was receiving the infusions at the blood center and one presented severe clinical complications, requiring frequent changes in the infusion scheme and, for these reasons, they were excluded from the study. Thus, those responsible for the prophylaxis of 34 patients and five observers were recruited for the research, but two patients declined participation. The 32 patients, whose prophylaxis adherence was evaluated, were all males, 27 diagnosed with hemophilia A (21 with factor VIII dosage <1%) and five with hemophilia B (four with factor IX dosage <1%). The patients’ ages ranged from three to 60 years (mean of 20.6 years, with a standard deviation of 14.1). All the boys under the age of six were regularly attending preschool. The patient was responsible for the infusions in only 11 cases (34.3%). Tables 1 and 2 show the sociodemographic and clinical characteristics of the patients, respectively.
Sociodemographic characteristics of patients with hemophilia in a home prophylaxis regimen between October 2015 and November 2016.
| Sociodemographic characteristic | |
|---|---|
| Age (years) – mean (range) | 20.6 (3–60) |
| Self-declared color – n (%) | |
| White | 20 (62.5) |
| Black | 6 (18.8) |
| Mulatto | 4 (12.5) |
| Indigenous | 2 (6.3) |
| Marital status – n (%) | |
| Single | 28 (87.5) |
| Married | 2 (6.3) |
| Divorced | 2 (6.3) |
| Schooling of over 18-year olds – n (%) | |
| Higher education completed or in progress | 9 (56.4) |
| High school diploma | 1 (6.3) |
| Complete elementary education | 1 (6.3) |
| Incomplete elementary education | 4 (25) |
| No education at school | 1 (6.3) |
| Occupation of those over 18 – n (%) | |
| Student | 7 (43.8) |
| Retired due to disability | 6 (37.5) |
| Formal employee | 2 (12.5) |
| Unemployed | 1 (6.3) |
| Occupation of those under 18 – n (%) | |
| Student | 15 (93.8) |
| No occupation | 1 (6.2) |
Clinical characteristics and therapeutic aspects of hemophilia patients between October 2015 and November 2016.
| Clinical characteristic | |
|---|---|
| Type of hemophilia – n (%) | |
| A | 27 (84.4) |
| B | 5 (15.6) |
| Severity – n (%) | |
| <1% Factor VIII or IX | 25 (78.1) |
| 1–2%b Factor VIII or IX | 7 (21.9) |
| Family history of hemophilia – n (%) | |
| Yes | 18 (56.3) |
| No | 14 (43.8) |
| Presence of target joints before prophylaxis started – n (%) | |
| Yes | 28 (87.5) |
| No | 4 (12.5) |
| Clinically evident arthropathy – n (%) | |
| Yes | 18 (56.3) |
| No | 14 (43.8) |
| Physiotherapy in the past three months – n (%) | |
| Yes | 5 (15.6) |
| No | 27 (84.4) |
| Engage in regular physical activity – n (%) | |
| Yes | 20 (62.5) |
| No | 12 (37.5) |
| Time in prophylaxis – median (range) | 21.6 months (6.5–40.6) |
| Spontaneous hemarthrosis in the past three months – n (%) | |
| None | 20 (62.5) |
| 1 or 2 | 9 (28.2) |
| 3 or more | 3 (9.4) |
| Primary infuser – n (%) | |
| Patient | 11 (34.4) |
| Mother/father | 8 (25) |
| Sister/brother | 1 (3.1) |
| Other relative | 1 (3.1) |
| Health professional | 11 (34.4) |
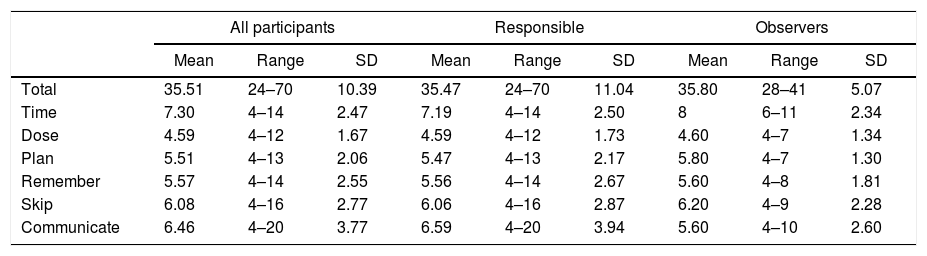
The total mean of the VERITAS-Pro was 35.51 (range: 24–70) when the responses of those responsible for prophylaxis and the observers were analyzed together. There was no statistically significant difference when the scores were evaluated separately (35.47 and 35.8, respectively; p-value=0.914). There was also no difference when the patients themselves were the primary infusers (38.91) or others took on this responsibility (33.81; p-value=0.17). The averages of the subscales ranged from 4.59 (Dose) to 7.3 (Time) (Table 3).
VERITAS-Pro total and subscale scores.
| All participants | Responsible | Observers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | SD | Mean | Range | SD | Mean | Range | SD | |
| Total | 35.51 | 24–70 | 10.39 | 35.47 | 24–70 | 11.04 | 35.80 | 28–41 | 5.07 |
| Time | 7.30 | 4–14 | 2.47 | 7.19 | 4–14 | 2.50 | 8 | 6–11 | 2.34 |
| Dose | 4.59 | 4–12 | 1.67 | 4.59 | 4–12 | 1.73 | 4.60 | 4–7 | 1.34 |
| Plan | 5.51 | 4–13 | 2.06 | 5.47 | 4–13 | 2.17 | 5.80 | 4–7 | 1.30 |
| Remember | 5.57 | 4–14 | 2.55 | 5.56 | 4–14 | 2.67 | 5.60 | 4–8 | 1.81 |
| Skip | 6.08 | 4–16 | 2.77 | 6.06 | 4–16 | 2.87 | 6.20 | 4–9 | 2.28 |
| Communicate | 6.46 | 4–20 | 3.77 | 6.59 | 4–20 | 3.94 | 5.60 | 4–10 | 2.60 |
SD: standard deviation.
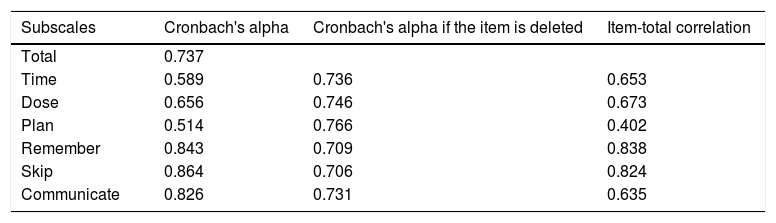
Results showed a very good internal consistency for the VERITAS-Pro total score; excellent for the Remember, Skip and Communicate subscales; good for the Dose subscale; and minimally acceptable for the Time and Plan subscales (Table 4).
Internal consistency analysis of the Brazilian version of the VERITAS-Pro.
| Subscales | Cronbach's alpha | Cronbach's alpha if the item is deleted | Item-total correlation |
|---|---|---|---|
| Total | 0.737 | ||
| Time | 0.589 | 0.736 | 0.653 |
| Dose | 0.656 | 0.746 | 0.673 |
| Plan | 0.514 | 0.766 | 0.402 |
| Remember | 0.843 | 0.709 | 0.838 |
| Skip | 0.864 | 0.706 | 0.824 |
| Communicate | 0.826 | 0.731 | 0.635 |
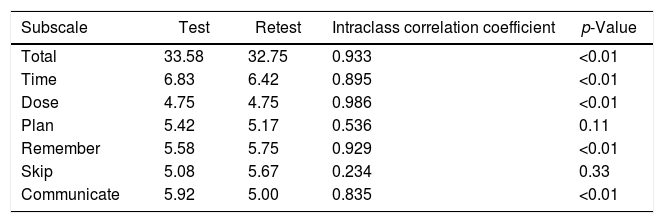
Twelve participants (37.5%) agreed to answer the VERITAS-Pro questionnaire on more than one occasion for test–retest reliability analysis. The mean interval between the two responses was 55.5 days (standard deviation of 11.9 days; range: 24–145 days). The ICC for the test–retest was excellent for the total score (0.933; p-value <0.01), and for all subscales except for the Plan and Skip domains (Table 5).
Evaluation of reproducibility of the Brazilian version of the VERITAS-Pro.
| Subscale | Test | Retest | Intraclass correlation coefficient | p-Value |
|---|---|---|---|---|
| Total | 33.58 | 32.75 | 0.933 | <0.01 |
| Time | 6.83 | 6.42 | 0.895 | <0.01 |
| Dose | 4.75 | 4.75 | 0.986 | <0.01 |
| Plan | 5.42 | 5.17 | 0.536 | 0.11 |
| Remember | 5.58 | 5.75 | 0.929 | <0.01 |
| Skip | 5.08 | 5.67 | 0.234 | 0.33 |
| Communicate | 5.92 | 5.00 | 0.835 | <0.01 |
The infusion logs of 32 patients were evaluated regarding the 12 weeks that preceded the application of the VERITAS-Pro. In all cases it was not possible to identify prophylactic infusions done outside the schedule of episodic infusions for the treatment of hemorrhagic events. Those responsible reported an average infusion rate of 72.17% of the prophylactic doses prescribed in the period (range: 25–105.5%; standard deviation: 25.8%). Half of those responsible for prophylaxis reported infusion of 80% or more of the prescribed doses, which characterizes good adherence, when this is evaluated dichotomously.7,10,15,16
Adherence measured by visual analog scaleThe global adherence reported by 35 participants (31 responsible for infusions and four observers) through the analog scale ranged from 6 to 10. Mean global adherence was 9.2 with a standard deviation of 1.05 and a median of 10.
Adherence measured by the blood center pharmacy's dispensing of factor concentrateThe blood center pharmacy usually dispenses doses sufficient for four weeks of prophylaxis on each visit of those responsible for infusions. Thus, the individuals responsible are expected to return with the used bottles at the end of this period and replenish their household supply. The dispensing of doses sufficient for prophylactic infusions in the 12 weeks prior to the survey was analyzed, and the actual time until the return of all used bottles was computed. Adherence was calculated by dividing the number of doses dispensed by the number of doses required to meet the prescription until the return, revealing a mean adherence of 89.02% (standard deviation of 18.3, with a minimum of 45.9% and a maximum of 130%).
Concurrent validity of the VERITAS-ProSince better adherence is characterized by lower scores on the VERITAS-Pro, higher scores on the visual analog scale, and by higher adherence percentages based on pharmacy dispensing and on infusion logs, negative correlations indicate a stronger correspondence of VERITAS-Pro with other adherence measures. Therefore, VERITAS-Pro scores were moderately correlated with the global adherence scale (r=−0.529; p-value=0.002) and with the records in infusion logs (r=−0.516; p-value=0.003), but showed a weak correlation with the pharmacy dispensing records (r=−0.32; p-value=0.074).
DiscussionProphylaxis is considered the standard therapy for patients with severe hemophilia17 as it is capable of preventing arthropathy when begun early,18 and of reducing the number of bleeding episodes, and improving the quality of life of individuals who already have irreversible joint damage.19 However, adherence to treatment is essential to achieve these results.20,21
In Brazil, the prophylactic treatment regimens for hemophilia were incorporated by the Brazilian National Health System as of November 2011.22 The lack of a reliable instrument to assess adherence to prophylaxis has been one of the obstacles to research in this field since then.23 To meet this need, the psychometric properties of the Brazilian version of the VERITAS-Pro were evaluated, showing good reliability and validity when applied to both those responsible for prophylaxis and to observers of the treatment carried out.
The total score showed a very good internal consistency, although lower than the original questionnaire (α=0.737 versus α=0.92). In addition, the scores had a moderate correlation with the visual scale of adherence and with the percentage of administered infusions recorded in the patients’ logs. That is, the Brazilian version of the VERITAS-Pro is a measure with temporal stability and that relates with other measures of adherence to prophylaxis. Almost all the subscales showed good internal consistency, except for the Time (α=0.589) and Plan subscales (α=0.514). Particular attention should be paid to these subscales and items considering these subscales in future analysis because their modification could improve the quality of the scale. Unfortunately, a factorial analysis was not possible due to the size of the sample.
The total score and all the subscales showed excellent test–retest reliability, except for the Skip subscale (ICC=0.234), whose means increased significantly in the participant's second assessment. This increase, which translates as worsening adherence in the Skip domain, was unexpected since individuals tend to improve their behavior, albeit transiently, after being evaluated for the first time.24
As in the original study, the Dose subscale exhibited the lowest mean while the Time subscale presented the highest, indicating that participants reported better adherence in administering the correct dose and worse in administering it on prescribed days and times.12
In addition, as in the American work, the dispensing of factor concentrates by the pharmacy was a less useful validation measure than the infusion logs and the visual scale of adherence.12 This is explained by the fact that the dispensing of medications includes variables that are not directly related to patient adherence, such as the healthcare model of the local health system. Cuesta-Barriuso et al. argue that free treatment can weaken the patient's commitment in following through with the prescriptions, but not in the acquisition of the medication.25 While the infusion logs represent an excellent source of data for validation and are commonly used to monitor infusions at home, they only provide quantitative data.3,7,15,26
The superiority of the VERITAS-Pro over the global measures is due to its ability to recognize the multiple facets of the adherence construct, represented by the subscales, and to perceive different types of behavior. While the Time, Dose and Skip subscales identify whether and when infusions were administered, the Plan and Communicate domains reveal the individual's baseline behavior. This more comprehensive view provided by the VERITAS-Pro assists in directing interventions to improve adherence to prophylaxis.12
Another advantage of the Brazilian version of the VERITAS-Pro was that it is easy to understand and quick to complete, making it suitable for routine use in the clinical practice.
It is believed that this is the first work in Brazil on adherence to prophylaxis in hemophilia. Although small, the sample was sufficient for the validation analyses of the Brazilian version of the VERITAS-Pro. Unfortunately, subgroup analyses were limited by the low number of participants. Although differences in adherence were not described according to the type or severity of hemophilia, or regarding the presence of infections such as hepatitis C or human immunodeficiency virus,25 Miebach and Kalnins reported that adherence varies according to age group and is lower among young adults.27 Studies with larger populations are needed for these evaluations in the Brazilian context.
Finally, it should be noted that although all the children with severe hemophilia were in prophylaxis during this study, the situation of the adults was quite different. The results of this study showed that patients in the Brazilian sample had higher adherence to treatment than those enrolled in the study conducted by Duncan et al.,12 but a significant percentage chose to continue with episodic treatment, expressing the lowest possible level of adherence to prophylaxis, yet these patients were not considered by the VERITAS-Pro.
ConclusionThe Brazilian version of the VERITAS-Pro is a quantitative and detailed instrument for assessing adherence to hemophilia prophylaxis. Evidence from this study supports its validity and reliability for use in the clinical and research contexts. This validated scale can aid in the understanding of behaviors and in the identification of modifiable determinants of non-adherence associated with unsatisfactory orthopedic outcomes. The findings derived from the VERITAS-Pro can facilitate the design of targeted interventions in these situations, but future studies are needed to confirm the relationship between adherence, patient characteristics, and clinical outcomes.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank Maria Cristina Belletti Rodrigues for her assistance with one of the translations of the original questionnaire.