As respiratory infections are the leading cause of morbidity and mortality in patients with chronic lymphocytic leukemia (CLL), routine vaccination against microbes like streptococcus pneumoniae is strongly recommended to decrease the chances of severe disease.1 However, multiple studies have previously shown poor seroconversion rates in response to immunization among CLL patients, particularly those with longstanding disease.2 The pathogenesis involves impaired signaling between B and T cells along with B-CLL mediated apoptosis, hypogammaglobulinemia, and dysfunctional cellular immunity.2 Over the last decade, Bruton Tyrosine Kinase Inhibitors (BTKi) have increasingly been used as the first-line treatment for CLL because of their ease of administration and better side effect profile.3 The effect of BTKi on humoral immunity and whether their use leads to an increased susceptibility to infections is unclear as only a few small studies have evaluated their impact on vaccine responses. To consolidate data for better understanding, the authors performed a thorough literature search of online medical libraries to collect studies relevant to the topic.
We searched PubMed, Google Scholar, And EmCare using the search terms ‘Leukemia, Lymphocytic, Chronic, B-Cell AND vaccines’ and ‘BTK protein, human AND vaccines’. All articles found were reviewed to gather studies evaluating specifically the vaccine responses in CLL patients on BTKi. Six studies (n= 411) met the inclusion criteria (Table 1).
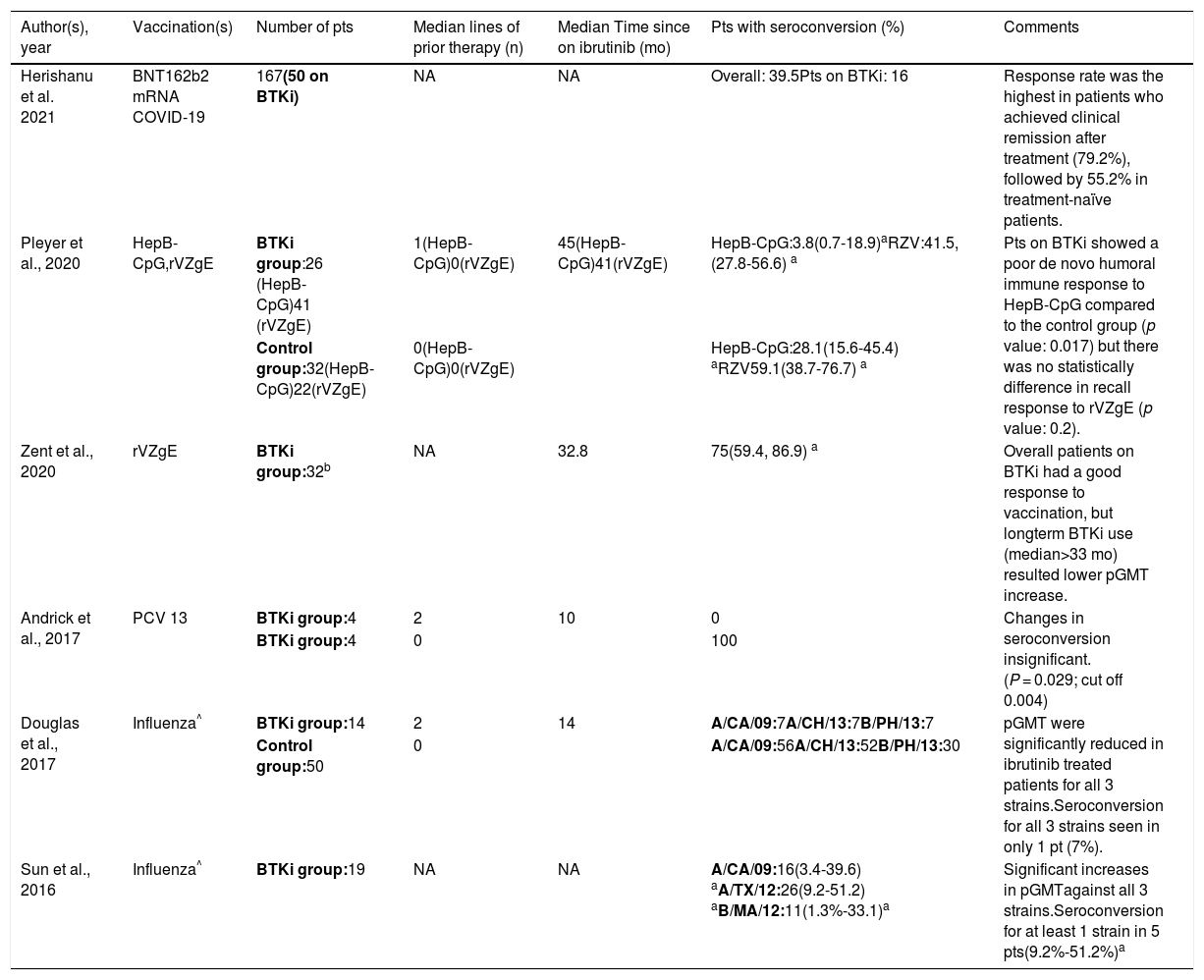
Summary of studies evaluating vaccine responses in patients with chronic lymphocytic leukemia on treatment with Bruton Tyrosine Kinase Inhibitors.
| Author(s), year | Vaccination(s) | Number of pts | Median lines of prior therapy (n) | Median Time since on ibrutinib (mo) | Pts with seroconversion (%) | Comments |
|---|---|---|---|---|---|---|
| Herishanu et al. 2021 | BNT162b2 mRNA COVID-19 | 167(50 on BTKi) | NA | NA | Overall: 39.5Pts on BTKi: 16 | Response rate was the highest in patients who achieved clinical remission after treatment (79.2%), followed by 55.2% in treatment-naïve patients. |
| Pleyer et al., 2020 | HepB-CpG,rVZgE | BTKi group:26 (HepB-CpG)41 (rVZgE) | 1(HepB-CpG)0(rVZgE) | 45(HepB-CpG)41(rVZgE) | HepB-CpG:3.8(0.7-18.9)aRZV:41.5,(27.8-56.6) a | Pts on BTKi showed a poor de novo humoral immune response to HepB-CpG compared to the control group (p value: 0.017) but there was no statistically difference in recall response to rVZgE (p value: 0.2). |
| Control group:32(HepB-CpG)22(rVZgE) | 0(HepB-CpG)0(rVZgE) | HepB-CpG:28.1(15.6-45.4) aRZV59.1(38.7-76.7) a | ||||
| Zent et al., 2020 | rVZgE | BTKi group:32b | NA | 32.8 | 75(59.4, 86.9) a | Overall patients on BTKi had a good response to vaccination, but longterm BTKi use (median>33 mo) resulted lower pGMT increase. |
| Andrick et al., 2017 | PCV 13 | BTKi group:4 | 2 | 10 | 0 | Changes in seroconversion insignificant.(P = 0.029; cut off 0.004) |
| BTKi group:4 | 0 | 100 | ||||
| Douglas et al., 2017 | Influenza^ | BTKi group:14 | 2 | 14 | A/CA/09:7A/CH/13:7B/PH/13:7 | pGMT were significantly reduced in ibrutinib treated patients for all 3 strains.Seroconversion for all 3 strains seen in only 1 pt (7%). |
| Control group:50 | 0 | A/CA/09:56A/CH/13:52B/PH/13:30 | ||||
| Sun et al., 2016 | Influenza^ | BTKi group:19 | NA | NA | A/CA/09:16(3.4-39.6) aA/TX/12:26(9.2-51.2) aB/MA/12:11(1.3%-33.1)a | Significant increases in pGMTagainst all 3 strains.Seroconversion for at least 1 strain in 5 pts(9.2%-51.2%)a |
n: number pts: patients, BTKi: Bruton Tyrosine Kinase Inhibitor, HepB-CpG: Hepatitis B vaccine, rVZgE: recombinant varicella zoster vaccine, pGMT: post vaccination geometric mean titer, mo: months, A/CA/09: A/California/7/2009, A/TX/12: A/Texas/50/2012, B/MA/12: B/Massachusetts/2/2012; A/CH/13: A/Switzerland/9715293/2013, B/PH/13: B/Phuket/3073/2013, PCV 13: pneumococcal conjugate vaccine 13, NA: Not available,
Initial studies done by Sun et al. and Douglas et al. revealed a notable difference in response to the influenza vaccine with BTKi use.4,5 While the former group observed at least seroconversion for one strain in 5 patients (26%) [95% confidence interval (CI) = 9.2%-51.2%; p-value: <0.002) in their single-arm study, only 1 patient (5%) mounted an adequate serological response in the latter group's study (p-value: 0.003 for A/California/7/2009 (H1N1), p-value: 0.007 for A/Switzerland/9715293/2013 (H3N2), and p-value: 0.16 for B/Phuket/3073/2013 (B/Yamagata lineage) compared to age-matched controls.4,5 While assessing the response to pneumococcal conjugate vaccine 13 (PCV 13) in CLL patients on BTKi, Andrick et al. demonstrated a marked reduction in seroconversion compared to the control group as none of the four patients on BTKi developed immunoglobulin titers in the protective range. All four CLL patients in the control group seroconverted (p-value: 0.029; cut off 0.004 post-hoc Fisher exact method).6 Zent et al. evaluated both humoral and cellular immune response to recombinant varicella-zoster glycoprotein E (rVZgE) vaccine in patients with CLL or lymphoplasmacytic lymphoma (LPL) on treatment with BTKi as the first line of therapy.7 Twenty-four patients (75%; 95% CI: 59.4-86.9%) had a four-fold increase in VZgE-specific IgG titers following vaccination. Of these 24 patients, 21 patients (87.5%) also showed an adequate CD4+ cellular immune response. The association between humoral and cellular immune response was significant with Fisher's exact test (p-value: 0.047).7 Pleyer et al. compared the vaccine response to hepatitis B vaccine (HepB-CpG) and rVZgE vaccine among CLL patients on BTKi versus treatment-naïve controls.8 The results showed a significantly lower response to HepB-CpG in the experimental arm as only one patient (3.8%; 95% CI: 0.7-18.9%) had an adequate rise in anti-HbS IgG specific titers compared to 9 patients (28.1%; 95%CI, 15.6-45.4%) in the control group (p-value: 0.017). Like in the study by Zent et al., the response to rVZgE vaccine was preserved, and there is no statistical difference between the two groups as 17 patients in the BTKi group (41.5%; 95% CI, 27.8-56.6%) and 13 patients (59.1%; 95% CI: 38.7–76.7%) generated a satisfactory rise in VZgE-specific IgG titers (p value: 0.2).8 Lastly, a study by Herishanu et al. showed a poor response to the BNT162b2 mRNA COVID-19 vaccine in CLL patients on treatment with BTKi as only eight patients (16%) had seroconversion 2–3 weeks after receiving the two doses of vaccine.9
Although some studies show poorer rates of seroconversion in patients on BTKi, it is well-known that treatment with anti-CD20 drugs like rituximab can lead to prolonged suppression of B-cell function.10 As these drugs were previously a part of the first-line treatment of CLL, lingering effects from their use may potentially affect the outcomes of studies. Sun et al. reported a satisfactory response to the influenza vaccine among patients on BTKi, however, data regarding prior exposure to anti-CD20 agents were not provided.4 Conversely, the study population for both Douglas et al. and Andrick et al. had extensive exposure to prior lines of therapies, albeit both excluded patients with exposure to anti-CD20 antibodies within six months before enrollment.5,6 None of the patients who received anti-CD20 treatment within a year responded to the COVID-19 vaccine in the study by Herishanu et al. either.9 A possible explanation postulated by the authors for poorer seroconversion rates in CLL patients on BTKi was the overexpression of SAMSNI gene as a downstream effect of BTK inhibition, leading to low IgM production levels.6 Prolonged exposure to BTKi may lead to a worsening of humoral immunity as the subgroup analysis of the study conducted by Zent et al. revealed a lower response rate in patients on BTKi therapy for a median of >40 months.7 Interestingly, newer studies have demonstrated a particularly blunted response to de novo antigens. First reported by Pleyer et al. with Hep-CpG and later by Herishanu et al. with the novel COVID-19 mRNA vaccine, the authors recommend considering vaccination before initiation of treatment for optimal results.8,9
Most of these studies have a small sample size which makes drawing conclusions regarding the effects of BTKi on humoral immunity challenging, and although larger studies are still needed to better elucidate the effects of BTKi on vaccination responses, for now it is prudent to advise patients to get vaccinated against respiratory pathogens including COVID-19 at the time of diagnosis as studies have consistently shown better seroconversion in treatment naïve patients.10