The myelodysplastic syndrome (MDS) represents a group of hematopoietic neoplasms that is characterized by clonal hematopoiesis, cytopenia and abnormal cellular maturation. Red cell distribution width (RDW) refers to the variation degree of erythrocyte size and it is a reflection of anisocytosis. Higher values have been linked to adverse outcomes, such as increased mortality, vascular events, kidney and liver disease and demonstrated to harbor poor prognosis in solid and hematological malignancies. The RDW value can be used as a contributing parameter for MDS diagnosis, as well as its prognosis. In this study, we essentially aimed to demonstrate the correlation between the RDW and MDS prognostic indexes.
Materials and methodsNinety-four MDS patients at the Aydın Adnan Menderes University Hematology Division were included in the study. The correlations between the RDW and laboratory values (either lactate dehydrogenase, albumin, globulin or ferritin) and the RDW prognostic scoring indexes (IPSS, WPSS, IPSS-R and LR-PSS) were investigated. The PASW for Windows, version 21.0 (SPSS Inc., Chicago, IL, USA), was used for statistical assessment. A p-value below 0.05 was the cut-off for the statistical significance.
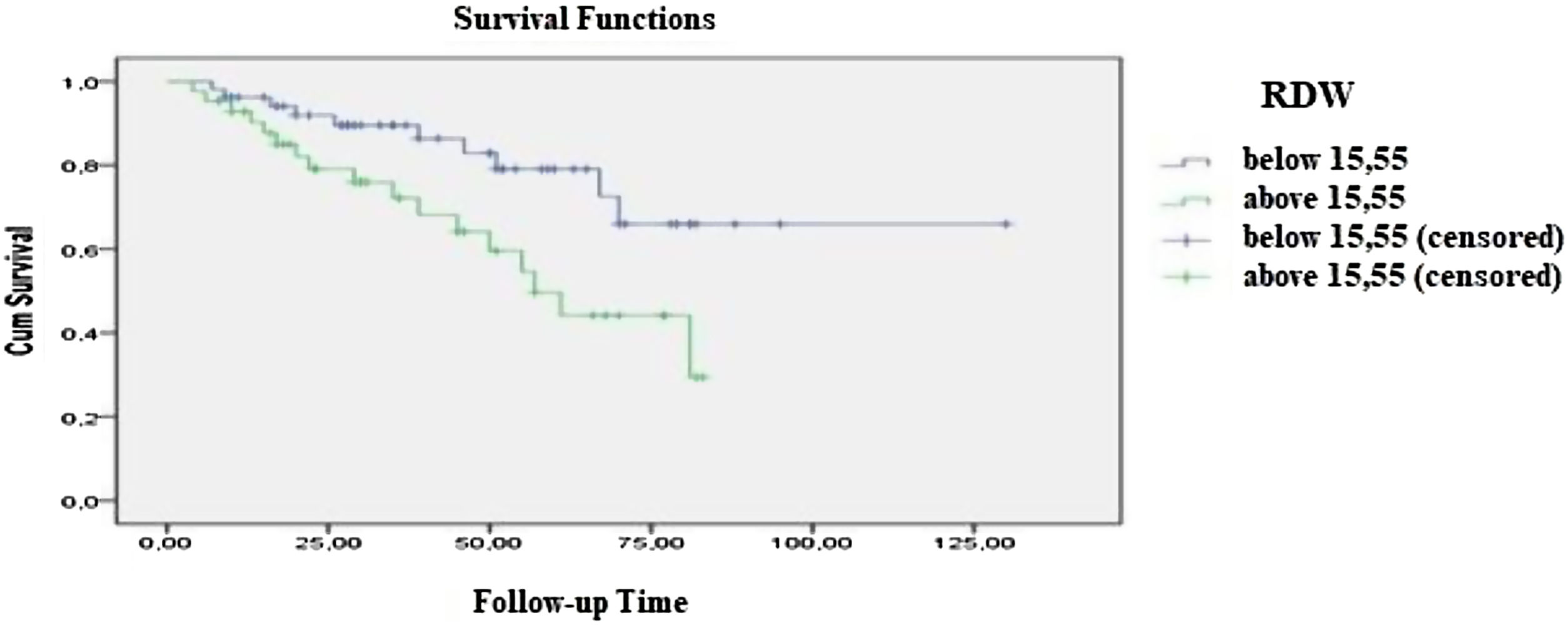
ResultsThe mean age of all the patients was 73 ± 10 years. Patients were observed for 41.88 ± 25 months. The mean RDW value for all cases was 15.5 ± 2.39. We found a statistically significant difference of survival between RDW values below and above 15.5% (p = 0.016). A significant difference was also observed according to the prognostic scoring indexes (see below).
ConclusionAn increase in RDW is probably related to dysplasia in the MDS and this constitutes a possible explanation for the poor outcome. Prognostic indexes might incorporate the RDW as a parameter in the future.
The myelodysplastic syndrome (MDS) is a group of myeloid neoplasms characterized by clonal hematopoiesis, cytopenia and abnormal cellular maturation. Besides transforming acute myeloblastic leukemia (AML), the MDS has clinical features, such as isolated cytopenias, bicytopenia or pancytopenia, which make the patient prone to bleeding and/or infections, as well as to anemia-related symptoms.1 The red cell distribution width (RDW) is a measure of the variation in RBC sizes and reflects anisocytosis. It is used to distinguish various types of anemia. An RDW elevation may be associated with increased overall mortality, cardiovascular and cerebrovascular events, venous thromboembolism and chronic kidney and liver disease.2–6 Hematological malignancies, such as chronic lymphocytic leukemia, chronic myeloid leukemia, diffuse large B cell lymphoma, NK/T cell lymphoma and multiple myeloma with higher RDW values have been demonstrated to harbor poor prognosis.7–9 An RDW increase can suggest the depth of dyserythropoiesis in the MDS. It has been shown to have prognostic significance, especially in low-risk MDS categories.10 This study essentially investigates the association between the RDW value and prognostic scoring systems in MDS patients.
MethodsNinety-four patients diagnosed with MDS according to the World Health Organisation (WHO) 2016 myeloid neoplasms classification (WHO-2016-MNC) between August 2018 and August 2019 at the Aydın Adnan Menderes University Hematology Division were included in the study. The study was designed as single-center, multidisciplinary, analytic and cross-sectional. All procedures were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from the participants. The patients under 18 years of age or those with the MDS-AML transformation were excluded. The patient blood samples were collected in hemogram tubes containing ethylenediaminetetraacetic acid (EDTA) and transferred to the laboratory within one hour of the collection. The RDW value was evaluated using the Mindray BC-6800 Hemogram Device and normal reference values were determined to be between 11 and 16%. The RDW (as coefficient variation RDW-CV) was measured using the formula: RDW-CV = 1 standard deviation ÷ mean corpuscular volume × 100).11 The following were used to estimate the prognosis: the International Prognostic Scoring System (IPSS), bone marrow blast percentage, karyotype analysis and cytopenia parameters; the WHO Classification-based Prognostic Scoring System (WPSS), WHO category, karyotype analysis and transfusion requirement parameters; the Low Risk Prognostic Scoring System (LR-PSS), presence of poor cytogenetics, age, hemoglobin, platelet levels and bone marrow blast percentage parameters, and; the Revised IPSS (IPSS-R), bone marrow blast ratio, karyotype analysis and hemoglobin-platelet-absolute neutrophil level parameters.12 The PASW for Windows, version 21.0 (SPSS Inc., Chicago, IL, USA), was used for statistical assessment. The compatibility of continuous variables with the normal distribution was performed with visual (histogram and probability graphics) and analytical methods (Kolmogorov–Smirnov/Shapiro–Wilk tests). For the descriptive statistics, mean and standard deviation were used for the data fitting normal distribution, whereas the minimum, median and maximum were used for the non-fitting data. The discrimination between categorical variables was obtained using the Chi-square test. The ANOVA and Student’s-t tests were used for the continuous variables to compare parametric properties (pp) of independent groups, while for the non-parametric, the Mann Whitney U or Kruskal–Wallis tests were performed to compare patient data for which a normal distribution could not be assigned. The Friedman and T tests were used to compare dependent groups with pp, whereas the Wilcoxon and Spearman tests were used for those which did not have pp. The survival analysis was performed using the Kaplan-Meier test (Figure 1). A p-value below 0.05 was the cut-off for the statistical significance.
ResultsNinety-four patients diagnosed with MDS were involved. The number of female and male patients were 49 (52.1%) and 45 (47.9%), respectively. The mean age of all the patients was 73 ± 10 years (73.3 ± 8 and 73.5 ± 10 for females and males, respectively). There was no statistically significant difference in age distribution between males and females. Patients were observed for 41 ± 25 months. Sixty-seven (71.3%) patients were still alive, while 27 patients (28.7%) died. The cumulative survival rate (CSR) for the first year and for the 5th year were 94.6 ± 2% and 65.6 ± 6% respectively. Forty patients (42.5%) had single lineage dysplasia (SLD), 22 patients (23.4%) had multilineage dysplasia (MLD), 1 patient (1.06%) had MDS-Ring Sideroblasts (RS) with SLD, 2 patients (2.1%) had MDS-RS-MLD, 3 patients (3.2%) had MDS with deletion 5q abnormality, 14 patients (14.9%) had MDS-EB-1 and 12 patients (12.8%) had MDS-EB-2, according to the WHO-2016-MNC. The mean RDW value for all cases was 15. 5 ± 2.39. Fifty-two and 42 patients were below 15.5 and above 15.5, respectively. The number of living patients below and above 15.5 for the RDW were 42 (80.8%) and 25 (59.5%), respectively. The CSR for the first year was 96.2 ± 2.7% and for the fifth year, 79.1 ± 6.9% for RDW values below 15.5 (follow-up time: 101 ± 7 months), whereas for RDW values above 15.5 (follow-up time: 56 ± 4 months). The CSR for the first year was 85 ± 5.7 % and for the fifth year, 49.6 ± 10.0%. There was a statistically significant difference between the two groups (p = 0.016). The relationship between prognostic values and the RDW of the participants was also examined. The mean RDW value of the 42 patients with a low IPSS index was 14.6 ± 2%, whereas it was 16.4 ± 2% for the 30 patients with an intermediate-high IPSS index. The mean RDW value of the 45 patients with a low/very low WPSS index was 14.7 ± 2%, whereas it was 16.5 ± 2% for the 27 patients with an intermediate/high/very high WPSS index. The mean RDW value of the 9 patients in the 1st category of the LR-PSS was 14.1 ± 1%, whereas among the 44 patients in the 2nd category and the 19 patients in the 3rd category, it was 14.8 ± 2 and 17.2 ± 2%, respectively. The mean RDW level of the 47 patients with a very low/low IPSS-R index was 14.8 ± 2%, whereas it was 16.5 ± 2% in the 25 patients with an intermediate/high/very high IPSS-R index. A statistically significant difference was found between each of the two groups. (p = 0.001; 0.002 < 0.001; 0.002, respectively) (Table 1). No correlation between the RDW and the LDH, albumin, globulin or ferritin values was detected (p = 0.142, 0.229, 0.343 and 0.518, respectively). As the RDW values could be affected by the accompanying iron deficiency, the mean transferrin saturation was also calculated and detected as 45.6 ± 91.3%, thus reinforcing the reliability of the mean RDW value in this study. Twenty-two patients were excluded from the prognostic scoring because no karyotype analysis could be performed due to technical reasons. The laboratory parameters of the patients are shown in Table 2.
The relationship between prognostic indexes and the RDW.
| Prognostic scoring indexes | n | RDW (%) | p | |
|---|---|---|---|---|
| IPSSa | Low | 42 | 14.63 ± 2.35 | 0.001 |
| Intermediate/high | 30 | 16.49 ± 2.17 | ||
| WPSSa | Low/very low | 45 | 14.73 ± 2.28 | 0.002 |
| Intermediate/high/very high | 27 | 16.53 ± 2.33 | ||
| LR-PSSa | 1st Category | 9 | 14.13 ± 1.75 | 0.000 |
| 2nd Category | 44 | 14.86 ± 2.08 | ||
| 3rd Category | 19 | 17.26 ± 2.58 | ||
| IPSS-Rb | Low/very low | 47 | 14.30 ± 2.36 | 0.002 |
| Intermediate/high/very high | 25 | 16.40 ± 2.26 | ||
Laboratory parameters of patients.
| Parameters | n | Median (±SD) |
|---|---|---|
| Hemoglobin(gr/dl) | 94 | 9.8 ± 1.8 |
| Hematocrit (%) | 94 | 30.3 ± 5.6 |
| Leukocyte(10³/µL) | 94 | 5,625 ± 4,427 |
| Neutrophil(10³/µL) | 94 | 3,235 ± 3,875 |
| Lymphocyte(10³/µL) | 94 | 1,615 ± 739 |
| Eosinophil(10³/µL) | 94 | 90 ± 451 |
| Monocyte(10³/µL) | 94 | 390 ± 428.37 |
| Platelet(10³/µL) | 94 | 178,500 ± 164,441 |
| RDW(%) | 94 | 15.20 ± 2.39 |
| MCV(fL) | 94 | 91.15 ± 14.08 |
| LDH(U/L) | 94 | 198 ± 179 |
| Albumin(g/L) | 92 | 4.00 ± 4.20 |
| Globulin(g/L) | 92 | 3.10 ± 2.19 |
| Ferritin(ng/mL) | 92 | 145.51 ± 1,481.89 |
| Transferrin saturation(%) | 92 | 45.6 ± 91.3 |
The RDW value can be evaluated as a predictor of the MDS diagnosis or, as our study shows, it can be an indicator of poor prognosis among the various prognostic indexes (WPSS, IPSS, IPSS-R, and LR-PSS). Various studies have shown that the hypothesis of the RDW to be a supportive marker of the MDS diagnosis. In a study by Rauw et al., the RDW was postulated to have an independent predictive value of diagnosing MDS in patients with unexplained cytopenia(s). In this study, a novel scoring system which includes age, RDW, mean corpuscular volume (MCV) and lactate dehydrogenase (LDH) was developed to predict the MDS diagnosis in patients with unexplained cytopenia(s) or macrocytosis within a 3-year period. The probability of diagnosing MDS was 12% if all 4 parameters were all negative, whereas it increased to 48%, if 3 or more parameters were positive.13 There are also other studies supporting the use of the RDW in predicting the MDS diagnosis.14,15 Age, MCV, LDH and RDW were also used as independent predictive parameters in a study involving 73 MDS patients by Buckstein et al.15 Besides the predictive diagnostic significance of MDS, one study by Baba et al., investigated the relationship of the RDW with clinical outcomes. This study included the following groups of patients: 59 refractory anemia (RA), 8 refractory anemia with ringed sideroblasts (RARS), 30 refractory anemia with excess blasts (RAEB) and 4 refractory anemia with excess blasts in transformation (RAEB-T), according to the French-American-British (FAB) classification. The RDW cut-off value was determined as 15%. The poor prognostic significance of the RDW increase was observed in the RA group (p = 0.0086), compared to the RAEB or RAEB-T groups.10 Regarding the RDW as a prognostic value, another study by Zhongxun et al. established that patients with bone marrow blasts, especially those with less than 5%, have a poorer outcome if they have an increased RDW value (< 14.5%) at diagnosis.16 An elevated RDW among those diagnosed with age-related clonal hematopoiesis (ARCH) or TP53- or U2AF-mutated patients is found to carry an acute risk of AML transformation.17,18 In our study, the statistical significance of an RDW increase has been detected in all risk groups, calculated by the prognostic scoring systems (WPSS, IPSS, IPSS-R and LR-PSS). Lower RDW values were detected in low-risk patients than in those with an intermediate or high risk. This difference shows that the RDW, with its low cost and ease of use, can be used to estimate the MDS prognosis. As far as we know, this is the first study that evaluates the association of the RDW and prognostic scoring systems in MDS. The limitation of our study is that the number of patients with low scoring indexes are greater in number than those intermediate or high risk. The other limitation of our study which can be considered was the impossibility of evaluating the RDW prognosis correlation regarding the MDS categories (according to the WHO-2016-MNC) or the impact of the bone marrow blast ratio due to the limited number of patients.
ConclusionThe RDW is a simple, rapid and inexpensive test which measures red blood cell morphological alterations by automated blood count devices. An increase in the RDW is probably related to dysplasia in MDS.10 Current prognostic scoring indexes have not included the RDW as a criterium. Further RDW studies performed in the future might determine the association in high-risk MDS patients and illustrate the prognostic significance for those with low-risk MDS.
Ethical approvalThe ethical committee approval was obtained for the study (No: 2018/1455).
Conflicts of interestThe authors declare no conflicts of interest.
This study was performed in collaboration among all authors. N.A. designed the study, G.S. performed the statistical analysis, A.T. wrote the protocol and the first draft of the manuscript. İ.Y. and Z.B. managed the analyses of the study. A.T. and İ.Y. managed the literature searches. All authors read and approved the final manuscript