Lewis antibodies have been thought to play a small role in clinical transfusion practice, but recent reports suggest that they have gained more importance in the context of transfusion and transplantation. Data regarding the prevalence of Lewis antibodies and their clinical significance in the Indian context is very limited. Hence, this study was aimed at analyzing the serological characteristics and clinical significance of Lewis antibodies encountered in our patient and donor populations.
MethodsThe retrospective data analyzed the records of red cell antibody screening results and the additional serological evaluation performed on the donor and patient samples included in the study.
ResultsA total of 26 study subjects were noted to have Lewis antibodies (including 6 healthy donors and 20 patients). Of them, 13 individuals had anti-Leb, 10 had anti-Lea and the remaining three had an anti-Lea/Leb mixture. IgG Lewis antibodies were detected in 7 individuals. All cases of IgM Lewis antibodies detected were reacting at 37°C. Two patients were suspected of having hemolytic transfusion reactions due to Lewis antibodies. Antigen-negative cross-match compatible units were provided for transfusion in the recipients.
ConclusionLewis antibodies of the IgM class reacting at 37°C should be regarded as clinically important. The present study findings urge that the lab personnel look for the thermal amplitude of Lewis antibodies, irrespective of the fact that the antibody class and antigen-negative crossmatch compatible units should be provided to avoid hemolytic transfusion reactions.
Lewis antigens are widely distributed in the body and hence, they are called histoblood group antigens. Similar to ABH antigens, they are found in the pancreas, stomach, mucosa of small and large intestines, skeletal muscle, renal cortex and adrenal glands.1 Lewis antigens are believed to be produced by the exocrine glands of the small intestine and their synthesis is dependant on the presence of two enzymes encoded by the Se gene (FUT2) and Le gene (FUT3) located on chromosome 19p13.3 and 19q13.3, respectively.The Le gene produces a fucosyl transferase enzyme, which adds fucose directly to the type I precursor chain to form Lea. The presence of Se (H transferase) and Le genes eventually creates the Leb antigen by adding fucose to the type I H chain. Hence, Le(a-b+) individuals are always secretor and Le(a+b-) individuals are always non-secretors. Since red cells use type 2 chains, they cannot synthesize Lewis antigens, rather they are adsorbed from the plasma onto the red cell membrane.2
A total of 6 antigens (Lea, Leb, Leab, LebH, ALeb and BLeb) belong to the Lewis blood group system. Of them, Lea and Leb antigens are the most important and are frequently encountered in clinical practice.3 Accordingly, four Lewis antigen phenotypes have been described including Le(a-b-), Le(a-b+), Le(a+b-) and Le(a+b+). The frequencies of Lewis antigen phenotypes, including Le(a-b+), Le (a+b-), Le(a-b-) and Le(a+b+), among our studied donor population were 55%, 22.5%, 15.5% and 7%, respectively.
Lewis antibodies have been thought to play a small role in the clinical transfusion practice, but recent reports suggest that these antibodies have gained more importance in the context of transfusion and transplantation.4 Data regarding the prevalence of Lewis antibodies and their clinical significance in the Indian context is very limited. Hence, this study was aimed at analyzing the serological characteristics and clinical significance of Lewis antibodies encountered in our patient and donor populations.
Materials and methodsThe retrospective observational study was conducted in the Department of Transfusion Medicine in a tertiary care hospital in South India from February 2016 to February 2021. The institutional ethics committee approval was obtained. Antibody screening results of all the patient and donor samples performed during the study period were traced and the data regarding the samples having Lewis antibodies were compiled.
Blood grouping for all samples was routinely performed by automated blood grouping equipment (IH-500, BioRad, Cressier, Switzerland) in column agglutination technology (CAT), using monoclonal antisera. In the case of a blood group discrepancy, blood grouping was repeated, using the conventional tube technique with monoclonal antisera (Tulip Diagnostics, Goa, India) and in-house pooled cells. At our center, preliminary antibody screening using pooled O cells was performed on all the donor samples. Donor samples testing positive during preliminary screening and those patient samples with blood group discrepancy/crossmatch incompatibility/samples of all RhD negative pregnant women were subjected to antibody screening, using commercial cell panels (Diacell, Biorad, Switzerland) in CAT.
Antibody identification was subsequently performed to detect the specificity of antibodies, using a commercial 11-cell panel (Diapanel Biorad, Switzerland) in CAT. Papain-treated red cells were used to augment the agglutination reaction in CAT. The direct antiglobulin test (DAT) was performed on all samples (before performing antigen phenotyping), using the monospecific Coombs gel card (DC screening II, Biorad, Cressier, Switzerland). The Lewis antigen (Lea and Leb) phenotyping was performed in CAT, using a single antigen testing card, as per manufacturer instructions (ID card, Diaclon anti-Lea and anti-Leb, Biorad, Cressier, Switzerland). The dithiothrietol (DTT) treatment of the serum was performed as described in the AABB Technical manual to detect the class (IgM or IgG) of the Lewis antibodies. The secretor status of the individuals was determined with the hemagglutination inhibition test. All the abovementioned procedures were performed as per the standard protocols of our department. The data were tabulated and the calculations were made using Excel software.
ResultsDuring the study period, 48,236 donor samples and 7,864 patient samples were screened for unexpected antibodies. A total of 26 study subjects were noted to have Lewis antibodies, including 6 healthy donors (0.01%) and 20 patients (0.25%). Of these, 13 individuals had anti-Leb, 10 had anti-Lea and the remaining three had a mixture of anti-Lea/Leb. The serological characteristics of the Lewis antibodies among patients and donors have been studied separately.
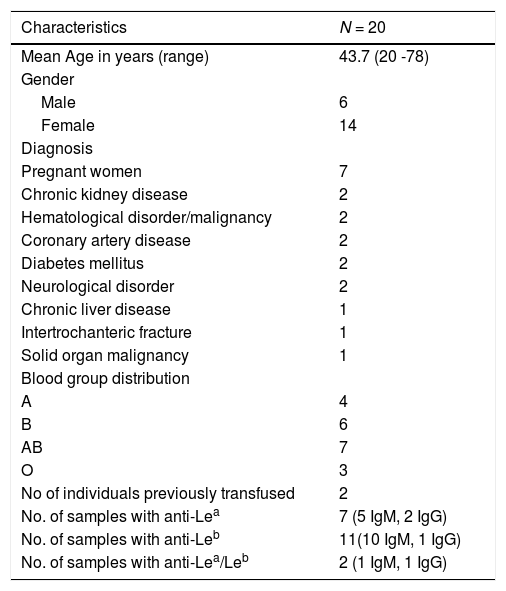
PatientsThe patient characteristics have been described in Table 1. Eleven patients had anti-Leb antibodies, anti-Lea was noted in seven patients and the remaining two had a mixture of anti-Lea/Leb antibodies. Of the study population, 35% (7 out of 20 patients) were pregnant women and two of them were multigravida. The individual clinical and laboratory characteristics have been summarized in Table 2. While a majority of them had IgM antibodies with a wide thermal amplitude (reacting at 4°C, 22°C and 37°C), the IgG class of Lewis antibodies were detected in four patients. Twelve patients were identified to be secretors. Two patients were typed as Le(a+b-), while most of them were phenotyped as Le(a-b-).
An outline of the demographics and serological characteristics of patients.
| Characteristics | N = 20 |
|---|---|
| Mean Age in years (range) | 43.7 (20 -78) |
| Gender | |
| Male | 6 |
| Female | 14 |
| Diagnosis | |
| Pregnant women | 7 |
| Chronic kidney disease | 2 |
| Hematological disorder/malignancy | 2 |
| Coronary artery disease | 2 |
| Diabetes mellitus | 2 |
| Neurological disorder | 2 |
| Chronic liver disease | 1 |
| Intertrochanteric fracture | 1 |
| Solid organ malignancy | 1 |
| Blood group distribution | |
| A | 4 |
| B | 6 |
| AB | 7 |
| O | 3 |
| No of individuals previously transfused | 2 |
| No. of samples with anti-Lea | 7 (5 IgM, 2 IgG) |
| No. of samples with anti-Leb | 11(10 IgM, 1 IgG) |
| No. of samples with anti-Lea/Leb | 2 (1 IgM, 1 IgG) |
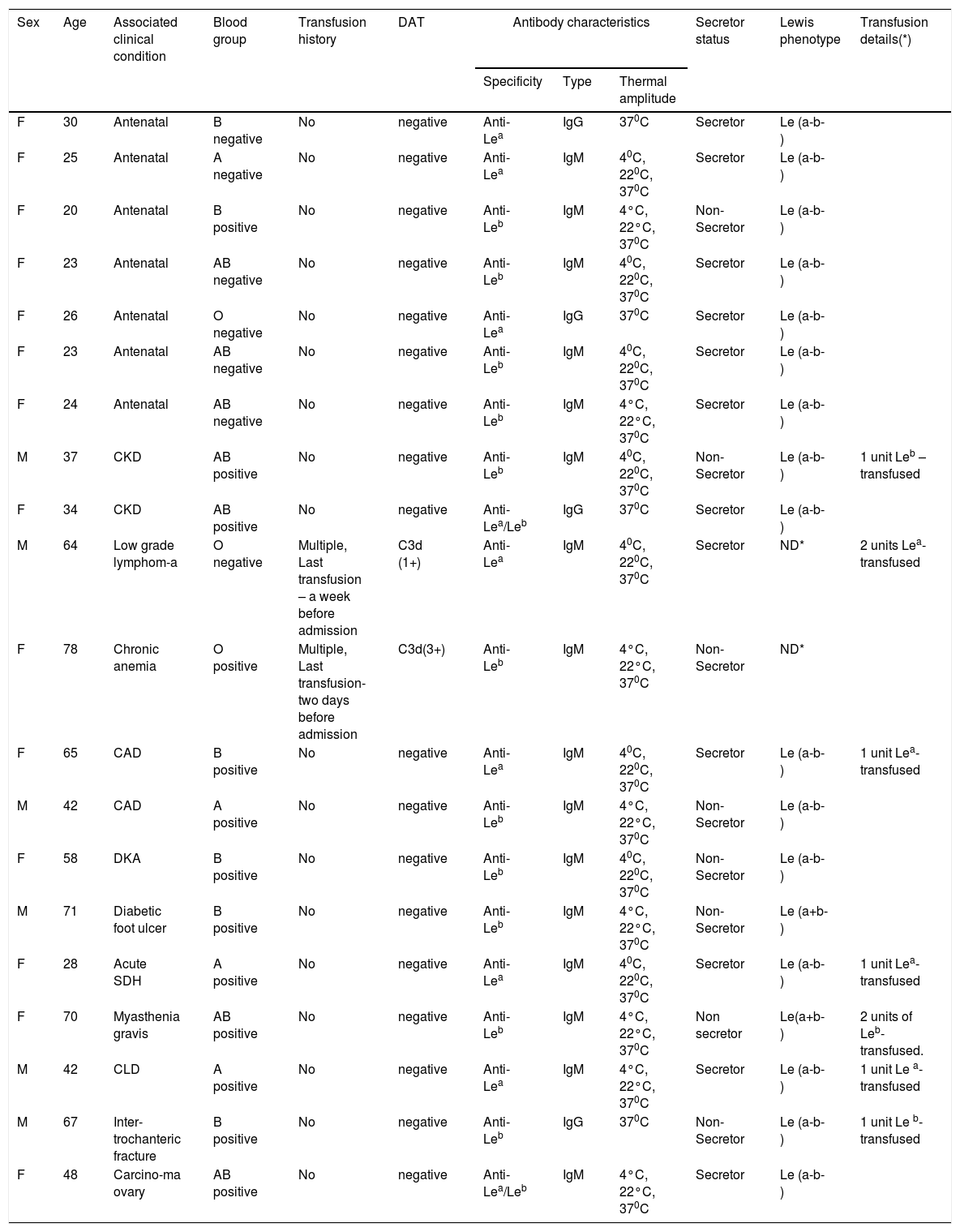
Individual data of the patients.
| Sex | Age | Associated clinical condition | Blood group | Transfusion history | DAT | Antibody characteristics | Secretor status | Lewis phenotype | Transfusion details(*) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Specificity | Type | Thermal amplitude | |||||||||
| F | 30 | Antenatal | B negative | No | negative | Anti-Lea | IgG | 370C | Secretor | Le (a-b-) | |
| F | 25 | Antenatal | A negative | No | negative | Anti-Lea | IgM | 40C, 220C, 370C | Secretor | Le (a-b-) | |
| F | 20 | Antenatal | B positive | No | negative | Anti-Leb | IgM | 4°C, 22°C, 370C | Non- Secretor | Le (a-b-) | |
| F | 23 | Antenatal | AB negative | No | negative | Anti-Leb | IgM | 40C, 220C, 370C | Secretor | Le (a-b-) | |
| F | 26 | Antenatal | O negative | No | negative | Anti-Lea | IgG | 370C | Secretor | Le (a-b-) | |
| F | 23 | Antenatal | AB negative | No | negative | Anti-Leb | IgM | 40C, 220C, 370C | Secretor | Le (a-b-) | |
| F | 24 | Antenatal | AB negative | No | negative | Anti-Leb | IgM | 4°C, 22°C, 370C | Secretor | Le (a-b-) | |
| M | 37 | CKD | AB positive | No | negative | Anti-Leb | IgM | 40C, 220C, 370C | Non- Secretor | Le (a-b-) | 1 unit Leb – transfused |
| F | 34 | CKD | AB positive | No | negative | Anti-Lea/Leb | IgG | 370C | Secretor | Le (a-b-) | |
| M | 64 | Low grade lymphom-a | O negative | Multiple, Last transfusion – a week before admission | C3d (1+) | Anti-Lea | IgM | 40C, 220C, 370C | Secretor | ND* | 2 units Lea-transfused |
| F | 78 | Chronic anemia | O positive | Multiple, Last transfusion- two days before admission | C3d(3+) | Anti-Leb | IgM | 4°C, 22°C, 370C | Non-Secretor | ND* | |
| F | 65 | CAD | B positive | No | negative | Anti-Lea | IgM | 40C, 220C, 370C | Secretor | Le (a-b-) | 1 unit Lea-transfused |
| M | 42 | CAD | A positive | No | negative | Anti-Leb | IgM | 4°C, 22°C, 370C | Non-Secretor | Le (a-b-) | |
| F | 58 | DKA | B positive | No | negative | Anti-Leb | IgM | 40C, 220C, 370C | Non-Secretor | Le (a-b-) | |
| M | 71 | Diabetic foot ulcer | B positive | No | negative | Anti-Leb | IgM | 4°C, 22°C, 370C | Non-Secretor | Le (a+b-) | |
| F | 28 | Acute SDH | A positive | No | negative | Anti-Lea | IgM | 40C, 220C, 370C | Secretor | Le (a-b-) | 1 unit Lea- transfused |
| F | 70 | Myasthenia gravis | AB positive | No | negative | Anti-Leb | IgM | 4°C, 22°C, 370C | Non secretor | Le(a+b-) | 2 units of Leb- transfused. |
| M | 42 | CLD | A positive | No | negative | Anti-Lea | IgM | 4°C, 22°C, 370C | Secretor | Le (a-b-) | 1 unit Le a- transfused |
| M | 67 | Inter-trochanteric fracture | B positive | No | negative | Anti-Leb | IgG | 370C | Non- Secretor | Le (a-b-) | 1 unit Le b- transfused |
| F | 48 | Carcino-ma ovary | AB positive | No | negative | Anti-Lea/Leb | IgM | 4°C, 22°C, 370C | Secretor | Le (a-b-) | |
*ND- Not done in view of recent transfusion history; DAT- Direct antiglobulin test; CKD- Chronic kidney disease; CAD- Coronary artery disease; DKA- Diabetic Ketoacidosis; SDH- Subdural hemorrhage; CLD- Chronic liver disease; * - PRBCs transfused
Except for two patients, none of them had been previously transfused. One patient, recently diagnosed with low-grade lymphoma, was referred to our hospital for further management. The patient had been transfused with 2 units of packed red blood cells (PRBCs) at another center, a week prior to admission. The hemoglobin (Hb) was 5.8g/dL and DAT was positive for C3d only(1+). The patient was found to have IgM class anti-Lea reacting at 37°C, while evaluating an incompatible crossmatch. Another elderly female received one unit of PRBCs, while being evaluated for anemia at a different center. Two days later, she was referred to our center because of the rapid decrease in Hb (from 6.2 to 4.8g/dL) post-transfusion. The DAT was positive for C3d (3+) only, with elevated serum lactate dehydrogenase levels and indirect hyperbilirubinemia. Anti-Leb antibodies of the IgM class, reacting at 37°C were detected, while investigating the transfusion reaction. The pre-transfusion sample could not be traced and hence, the source of the immunization could not be determined. Lewis phenotyping could not be performed in both of the cases in view of the recent transfusion and the possibility of the Lewis-antibody-induced delayed hemolytic transfusion reaction was considered.
In total, 7 patients who required transfusion were transfused with 9 Lewis-antigen negative compatible units and the transfusion episodes were uneventful. Individual characteristics of the patients are shown in Table 2.
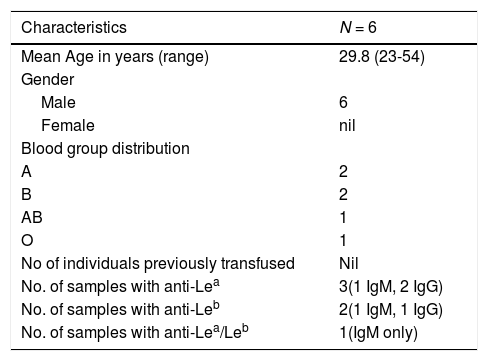
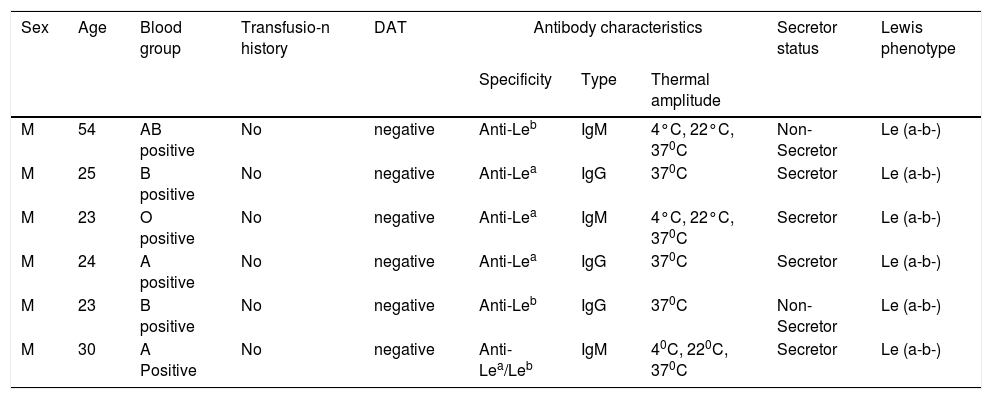
DonorsAmong the donors (n = 6), 3 had anti-Lea, 2 had anti-Leb, while one had a mixture of anti-Lea/Leb. All were males, aged from 23 to 54 years (Table 3). IgG class antibodies were detected in three donors and the remaining had IgM class antibodies reacting at 37°C. Two of them were non-secretors. All donors were typed Le(a-b-). None of them had been transfused. Individual characteristics of the donors have been tabulated in Table 4.
An outline of the demographics and serological characteristics of donors.
| Characteristics | N = 6 |
|---|---|
| Mean Age in years (range) | 29.8 (23-54) |
| Gender | |
| Male | 6 |
| Female | nil |
| Blood group distribution | |
| A | 2 |
| B | 2 |
| AB | 1 |
| O | 1 |
| No of individuals previously transfused | Nil |
| No. of samples with anti-Lea | 3(1 IgM, 2 IgG) |
| No. of samples with anti-Leb | 2(1 IgM, 1 IgG) |
| No. of samples with anti-Lea/Leb | 1(IgM only) |
Individual data of the donors.
| Sex | Age | Blood group | Transfusio-n history | DAT | Antibody characteristics | Secretor status | Lewis phenotype | ||
|---|---|---|---|---|---|---|---|---|---|
| Specificity | Type | Thermal amplitude | |||||||
| M | 54 | AB positive | No | negative | Anti-Leb | IgM | 4°C, 22°C, 370C | Non-Secretor | Le (a-b-) |
| M | 25 | B positive | No | negative | Anti-Lea | IgG | 370C | Secretor | Le (a-b-) |
| M | 23 | O positive | No | negative | Anti-Lea | IgM | 4°C, 22°C, 370C | Secretor | Le (a-b-) |
| M | 24 | A positive | No | negative | Anti-Lea | IgG | 370C | Secretor | Le (a-b-) |
| M | 23 | B positive | No | negative | Anti-Leb | IgG | 370C | Non-Secretor | Le (a-b-) |
| M | 30 | A Positive | No | negative | Anti-Lea/Leb | IgM | 40C, 220C, 370C | Secretor | Le (a-b-) |
DAT- Direct antiglobulin test.
Those antibodies which can cause the hemolytic transfusion reaction (HTR), hemolytic disease of the fetus and newborn (HDFN) and reduced in-vivo survival of the transfused red cells are considered clinically significant. They are generally IgG antibodies reacting at 37°C.3 Lewis antibodies have been thought to lack significance and, considered benign due to the following reasons. Firstly, it is the soluble nature of the Lewis antigens which enables the donor red cells to shed their antigens. Secondly, these shed antigens often get neutralized by the recipient antibodies. Thirdly, these antibodies display a strong predilection for reacting at lower temperatures. Fourthly, Lewis antigens are weakly expressed by non-O group individuals when compared to O group individuals and hence, crossmatch incompatibility is not often encountered. Finally, Lewis antibodies are predominantly naturally occurring IgM antibodies and only occasional cases of the IgG class have been documented.3,5,6 In the present study, it was noted that, although the majority of the Lewis antibodies were of the IgM class, IgG class antibodies were not uncommon. In addition, all cases of IgM class Lewis antibodies displayed reactivity at 37°C, highlighting their clinical significance. Similar observations were made by Das et al., in which 4 out of 7 individuals had IgG class Lewis antibodies7. Rarely has transfusion-induced Lewis alloimmunization been described in the literature.8,9
At our center, antibody screening of the patient population is performed selectively, which could be the limitation of the present study. Active screening of all the patients would yield the actual prevalence of Lewis antibodies in this cohort. In one study, on screening 69,354 individuals, no case of anti-Leb was found.7 In the present study, anti-Leb was observed to be more frequent than anti-Lea. The frequency of Lewis antibodies therefore tends to vary among different studies.10 Interestingly, it has been shown that the ABO blood group system can influence the expression of Lewis antigens. Especially the O group individuals have been shown to carry a significant amount of Lewis antigens on their red cells, compared to A/B/AB group individuals, which may influence the severity of hemolysis.5,8 Accordingly, O group individuals are less likely to produce Lewis antibodies, as seen in the present study.
Overall, there are two noteworthy features of Lewis antibodies which render them clinically relevant. 1, The hemolytic potential of Lewis antibodies has been ascribed to their complement-fixing ability, particularly the IgM class. The resultant instant intravascular hemolysis can even be fatal. Both acute and delayed HTRs have been documented with Lewis antibodies. Recently published literature highlights that the IgM class of Lewis antibodies (both anti-Lea and -Leb) reacting at 37°C can lead to acute hemolytic reactions in transfused recipients.4 In the current study, delayed hemolytic transfusion reaction, secondary to a Lewis antibody, was suspected in two patients, which was corroborated by the laboratory features of hemolysis (one had anti-Lea and the other had anti-Leb, IgM class antibodies, C3d positive DAT) and a temporal association with transfusion. 2, Individuals with a pre-existing Lewis antibody, are known to exhibit an anamnestic reaction, subsequent to an antigen-incompatible transfusion.5,8
Lewis antibodies are often found in the individuals having the Le(a-b-) phenotype and in the sera of pregnant women.11 In a study on the rate of alloimmunization among 5,347 pregnant women, anti-D was the most frequent specificity observed (34.2%), followed by Lewis antibodies (17.7%).12 In the present study, Lewis antibodies were most commonly found in pregnant women among the patient population, all with the Le(a-b-) phenotype. Hammar et al. observed that the frequency of the Le(a-b-) phenotype was significantly higher in pregnant women at the time of delivery (36%) when compared to non-pregnant women (11%). The null phenotype can appear as early as the 24th week of pregnancy due to the fourfold increase in the lipoprotein concentration, leading to the redistribution of the Lewis glycolipids on red cells. However, it is reversible and the original phenotype can be detected by 3 to 6 weeks after delivery.13 Despite occurring frequently amongst antenatal women, Lewis antibodies have rarely been implicated in HDFN as most of them are of the IgM class and Lewis antigens are poorly expressed at birth owing to their gut immaturity.2,3,4 In the present study, no case of Lewis antibody-related HDFN was observed, even though IgG antibodies were demonstrated in the sera of pregnant women.
Lewis antigens are usually present on tubular cells of the kidney tissue and may get adsorbed onto the glomerular and endothelial cells. Lewis antigens may generate cell-mediated or humoral response of a cytotoxic nature. Spitalnik et al. observed that those recipients with anti-Lea or anti-Leb antibodies who received Lewis incompatible kidney allografts later manifested with a 100% allograft rejection. Lewis negative recipients may develop antibodies on exposure to the Lewis antigens on the donor graft.14 Bortanyska et al., observed humoral rejection of the renal allografts due to complement-fixing Lewis antibodies formed post-transplant. Although Lewis antigen matching is not routinely recommended for renal transplants, for those with a past history of renal allograft rejection due to Lewis antibodies, the ensuing renal transplantation should be matched for Lewis antigens with the recipient.15
To conclude, most of the blood centers generally ignore evaluating naturally occurring IgM antibodies. But, the growing evidence on the clinical significance of Lewis antibodies demands more attention, thanks to the sensitive platforms employed during pre-transfusion testing. The present study findings urge the lab personnel look for the thermal amplitude of Lewis antibodies, irrespective of the antibody class.9 IgM antibodies reacting at 37°C should be regarded as clinically important and antigen-negative crossmatch-compatible units should be provided to avoid hemolytic transfusion reactions.