Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel human coronavirus responsible for the coronavirus disease 2019 (COVID-19). The pandemic stage, has raised concerns about the possible risk that it might be transmissible by transfusion. This theoretical risk is further supported by reports of the detection of viral RNA in the blood of some infected individuals. Some studies suggest that some individuals who are eligible to donate blood are infected with SARS-CoV-2 but are asymptomatic/pre-symptomatic. Furthemore, the data suggest that very few of these asymptomatic/pre-symptomatic individuals are RNAemic.1–3
Here we report on two cases of patients who received platelets and red blood cells transfusions from an individual who was infected with SARS-CoV-2.
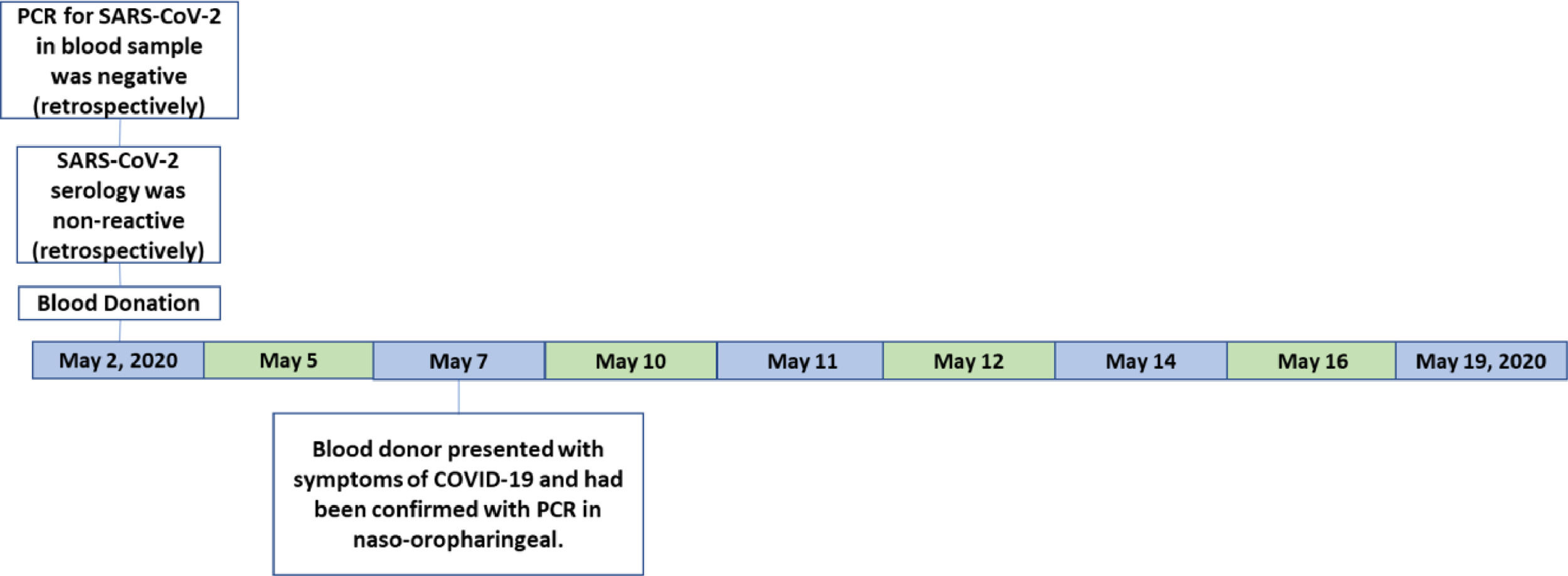
Blood donorA blood donation occurred on May 3, 2020. The blood donor presented with headache, cough, nasal congestion and runny nose on May 7, the day on which she was tested for SARS-CoV-2 by PCR of naso-oropharyngeal swabs (Veri-Q Prep M-16 extraction kit and Veri-Q PCR 316 detection kit). A day later, on May 8, she was informed that her test was positive and she notified the blood bank. The platelets and the red cell had been transfused into two distinct patients (patient 1 and patient 2). The plasma was discarded (Figure 1).
The blood sample was tested later by PCR (SARS-CoV-2 RNA extraction was performed on 300 µL of sera using the Extracta kit AN viral (Loccus) in an automated extractor (EXTRACTA 32, Loccus) following the manufacturer guidelines. The SARS-CoV-2-RT-PCR was conducted by Center for Disease Control (CDC) N1, N2 and RNAseP genes. The reaction protocol was performed using the GoTaq Probe 1-Step RT-qPCR System (Promega) according to the CDC protocol on the 7500 Real Time PCR System (Thermo Fisher Scientific). Cycles and temperatures for amplification were 30 min at 450 °C for reverse transcription, followed by 45 cycles at 95 °C for 2 min, 95 °C for 15 s and 60 °C for 1 min. For all reactions, negative and positive controls were added. The interpretation was made by the threshold cycle (Ct) and was negative.
Patient 1A 30-year-old woman diagnosed with B-cell acute lymphoid leukemia in November 2019 was submitted to haploidentical allogeneic hematopoietic stem cell transplantation after being treated with the GRAAPH protocol.4 After Day 12 of the transplantation (April 13), the patient presented with hemorrhagic cystitis, associated with a low platelet count, which were transfusion refractory. On the same day, a urine sample was collected to investigate for the Adenovirus and BK viruses. On April 27, a blood sample was collected to investigate for Adenovirus.
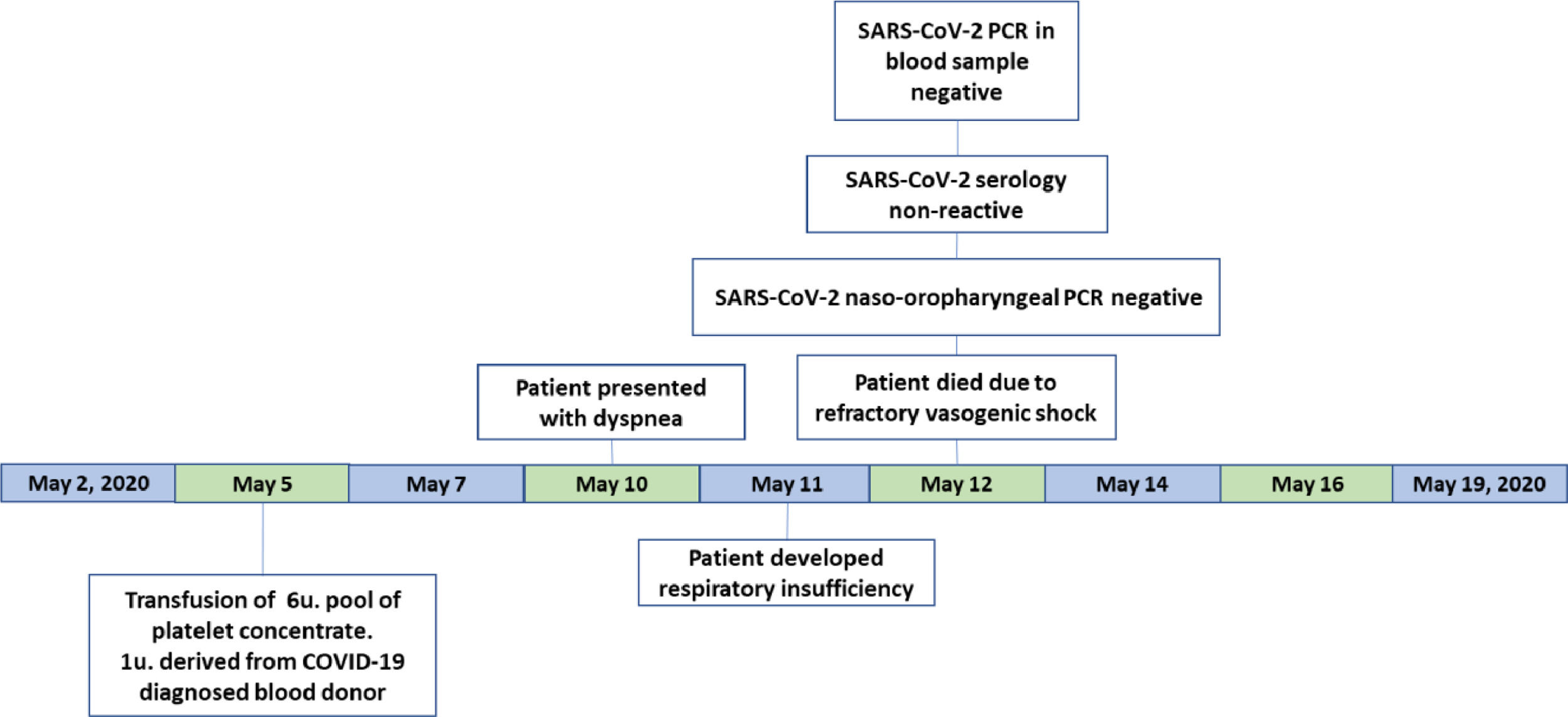
On May 5 (D+34 transplantation), she received a randomized platelet concentrate transfusion and 3 days later (May 8) the blood bank was notified that one of the blood donors had tested positive for SARS-CoV-2.
The patient was presenting intermittent fever for approximately 15 days with no hemodynamic instability, but five days after this platelet transfusion (May 10) she presented with dyspnea. The next day, May 11, the patient evolved to respiratory and renal failure. The assisting medical team collected blood samples for hemoculture and PCR for BK virus. They decided to place the patient on mechanical ventilation by an orotracheal tube. An antimicrobial schema containing meropenem, ertapenem, amikacin, polymyxin and linezolid was initiated. She developed vasogenic refractory shock on May 12, 2020 and died.
A naso-oropharyngeal swab and blood samples for SARS-CoV-2 investigation had been collected before the patient died. The PCR of naso-oropharyngeal swabs (Veri-Q Prep M-16 extraction kit and Veri-Q PCR 316 detection kit), the ELISA antibody detection tests (Architect® SARS-CoV-2 IgG assay (Abbott) and Elecsys® Anti-SARS-CoV-2 - Roche) and the blood PCR (described above) were negative (Figure 2).
The peripheral blood hemoculture showed Pseudomonas aeruginosa infection and the blood PCR for BK virus was positive, presenting a higher number of virus copies (3,400,000 copies), determining the cause of the hemorrhagic cystitis. Those results returned after the patient had died.
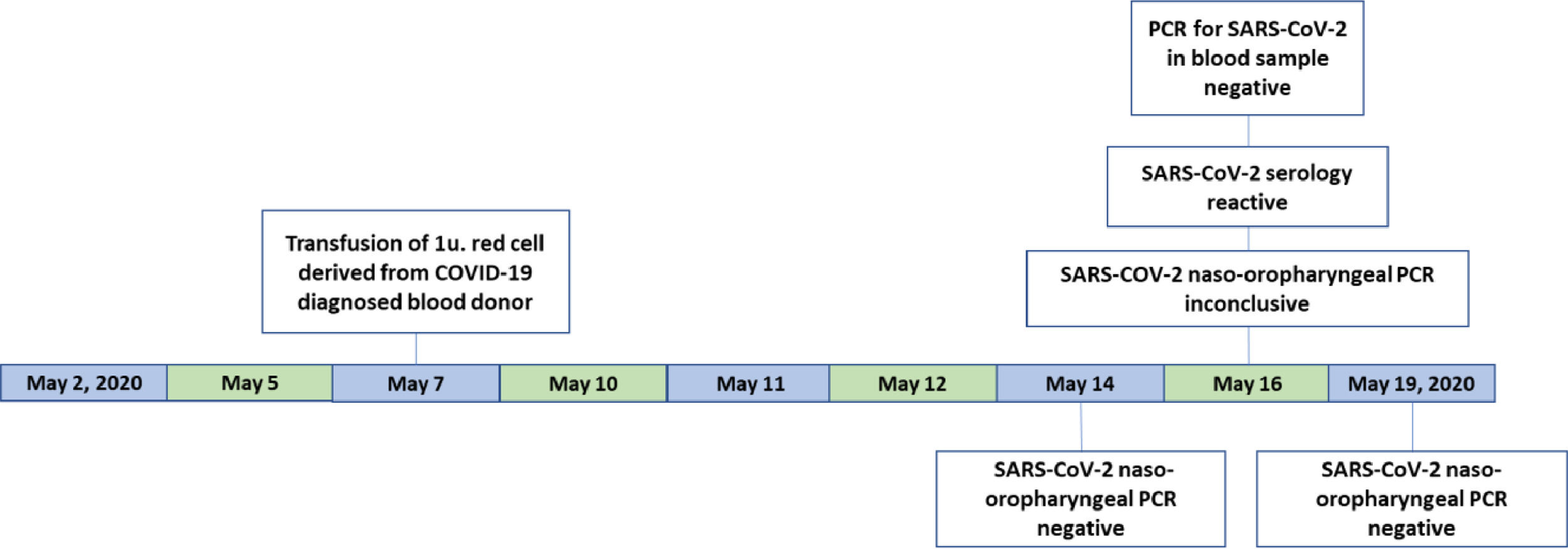
Patient 2A 20-year-old pregnant woman was admitted to the hospital in labor on May 2, 2020 and the childbirth occurred on the following day. The patient was kept hospitalized, presenting bleeding due to the childbirth. On May 7, a day before the notification from the blood donor, she received a red cell transfusion from the same blood donor that tested positive for SARS-CoV-2. She was discharged from the hospital on May 9.
On May 14, 16 and 18, she was subjected to SARS-CoV-2 testing by PCR with naso-oropharyngeal swabs (Veri-Q Prep M-16 extraction kit and Veri-Q PCR 316 detection kit). The first and the last PCR tests were negative, and the second test was inconclusive. The ELISA tests for COVID-19 (Architect® SARS-CoV-2 IgG assay (Abbott) and Elecsys® Anti-SARS-CoV-2 - Roche) were both reactive. The blood PCR (described above) was negative (Figure 3).
To date, the patient has not become symptomatic.
DiscussionCOVID-19 can be transmitted from asymptomatic individuals and the mean incubation period for SARS-CoV-2 infection has been reported to be from 0 to 14 days.5 These features may play a critical role in the possibility of transfusion transmission of the disease.
As stated by Katz,6 given that some asymptomatic/pre-symptomatic individuals appear to be infectious (through their respiratory secretions) and that some of these individuals must have donated blood since the beginning of the pandemic, if indeed SARS-CoV-2 were hematogenous, then it is likely that some cases of transmission by transfusion would have been identified among transfused patients on a worldwide scale.
On the other hand, to this day, there has not been a single reported case of a respiratory virus transmission by blood transfusion. Accordingly, the long historical track record on the mode of transmission of respiratory viruses predicts that SARS-CoV-2 would not be transmissible by transfusion. So far, this hypothesis appears to be true, as there has been no documented case of transfusion-transmitted SARS-CoV-2.
In the two cases reported on in the present study, transfusion of blood components from an infected donor who had not yet developed the signs and symptoms of COVID-19 did not result in disease transmission, even though the blood components recipients presented with a certain degree of immune suppression, one of them diagnosed with B-cell acute lymphoid leukemia and submitted to haploidentical allogeneic hematopoietic stem cell transplantation, while the other was a pregnant woman in labor.
Despite the fact that one of the patients presented repeatedly positive results for SARS-CoV-2 ELISA antibodies detection tests nine days after the transfusion of red blood cell from a donor infected with SARS-CoV-2, we cannot affirm that the source of infection had been the blood component transfusion.
Regarding the blood PCR, it is important to consider the assay limitations due to the use of sera as a template instead of naso-oropharyngeal samples. We have validated the use of serum in CDC 2019‐nCoV Real‐Time RT‐PCR Diagnostic Panel by amplification of RNase P.7 The limit of detection (LOD) for the CDC assay was determined to be 1 × 103 viral RNA genome equivalents per mL.8 Serum samples storage and handling were performed at -20°C and these should be considered.
It was not possible to proceed in the investigations, to determine if other family members had presented laboratory results reagent for SARS-CoV-2, or even if they had developed COVID-19, as the patient refused to permit further investigations.
SARS-CoV-2 infection can result in a spectrum of diseases, from mild respiratory symptoms to severe life-threatening pneumonia. Therefore, it is critical to consider the potential for transmission of this infection by blood transfusion.
Precautionary measures are recommended, such as deferral from donation of blood for 21 days after any possible exposure to patients with confirmed infection, as should those who are recovering from COVID-19 should avoid donating blood for at least 28 days after the resolution of their symptoms.9
The authors have no conflicts of interest to declare.