Real-world evidence on Mantle cell lymphoma (MCL) in Brazil is scarce. An observational study identified 183 MCL patients registered in the Hemato-Oncology Latin America Observational Registry. The five-year cumulative survival rate of MCL patients in Brazil was 35.4 %, the lowest rate among the five countries in Latin America, whose overall survival rate was 57.1 %.1 The use of rituximab shifted the treatment paradigm for MCL and has shown significant improvement in the treatment outcomes and overall survival in patients with MCL, both as first-line chemotherapy and as maintenance therapy.2,3 Ibrutinib, a Bruton tyrosine kinase (BTK) inhibitor, has also significantly impacted the treatment of relapsed and refractory (R/R) MCL, with studies showing high efficacy rates and improved outcomes for untreated MCL in select patients.4 Second-generation BTK inhibitors, such as acalabrutinib and zanubrutinib, are gaining attention for their selectivity and lower adverse events.5 However, these drugs are generally not available in Brazil's public healthcare system and were only recently approved for reimbursement by private healthcare agencies.
We report a real-world retrospective cohort in the pre-BTK inhibitor era seen at a single institution (HEMOMED) in Brazil between January 2009 and December 2020. This is the first real-world study investigating only MCL patients in the pre-BTK inhibitor era treated within the Brazilian private-health system. The inclusion criteria were adult patients aged ≥18 years diagnosed with MCL. All patients included were treatment-naïve. The authors excluded patients in other studies that prohibited them from participating in this observational study. This study was approved by the local Ethics Committees (CAAE number: 38,234,820.0.0000.0082).
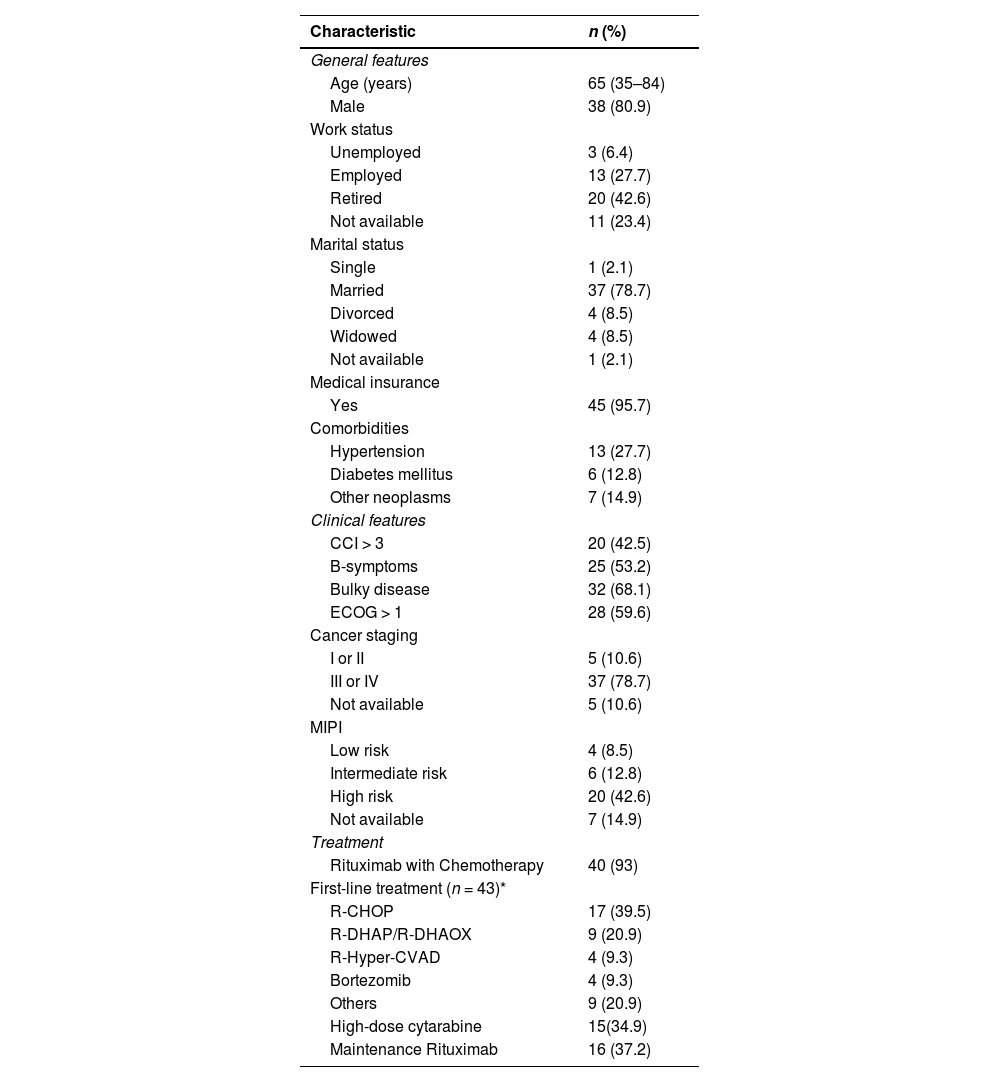
Forty-seven patients were included in the current study. Median age at diagnosis was 65 (range: 35–84) years. General patient characteristics are presented in Table 1. Indolent clinical presentation was observed in three (6.3 %), and watch and wait strategy was initially indicated in these cases. However, all these patients required treatment, with a median time to treatment (TTT) being 23.4 (95 % confidence interval – 95 % CI: 5.9–27.7) months. One patient (2.1 %) received an exclusively palliative regimen. Of the remaining 43 patients, 16 (37.2 %) were considered candidates for autologous stem cell transplant (ASCT). Of these 16 patients, seven were not submitted to ASCT consolidation: two failed to achieve objective response (OR) after induction, one died due to COVID-19 after C2 R-DHAOX (rituximab, dexamethasone, cytarabine, oxaliplatin), one refused the procedure due to the risk of COVID-19. Moreover, the ASCT team did not submit one patient to the procedure due to partial response, one was not submitted to the procedure due to a delay, whereas one case had stem cell mobilization failure after R-Hyper-CVAD (rituximab, cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride, methotrexate, cytarabine dexamethasone).
General characteristics of patients with mantle cell lymphoma (n = 47).
R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone; R-DHAP (rituximab dexamethasone cytarabine cisplatin); R-DHAOX (rituximab, dexamethasone, cytarabine, oxaliplatin); R-Hyper-CVAD (rituximab, cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride, methotrexate, cytarabine dexamethasone).
Data expressed as relative frequency (%) and median (interquartile range). *Percentages may not total 100 because of rounding or because some patients received more than one first-line therapy.
Among the 43 patients treated, 40 (93 %) patients received rituximab with chemotherapy; R- R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone) was the most frequently used treatment scheme in first-line (n = 17; 39.5 %), followed by R-DHAP (rituximab dexamethasone cytarabine cisplatin)/R-DHAOX (n = 6; 13.9 %), R-Hyper-CVAD (n = 6; 13.3 %) and bortezomib (n = 4; 9.3 %). In this group, 15 (34.9 %) were treated with high-dose cytarabine. After induction, complete response (CR) and OR were observed in 27 (62.7 %) and 31 (72 %) patients. Sixteen patients (37.2 %) received rituximab as maintenance (RM).
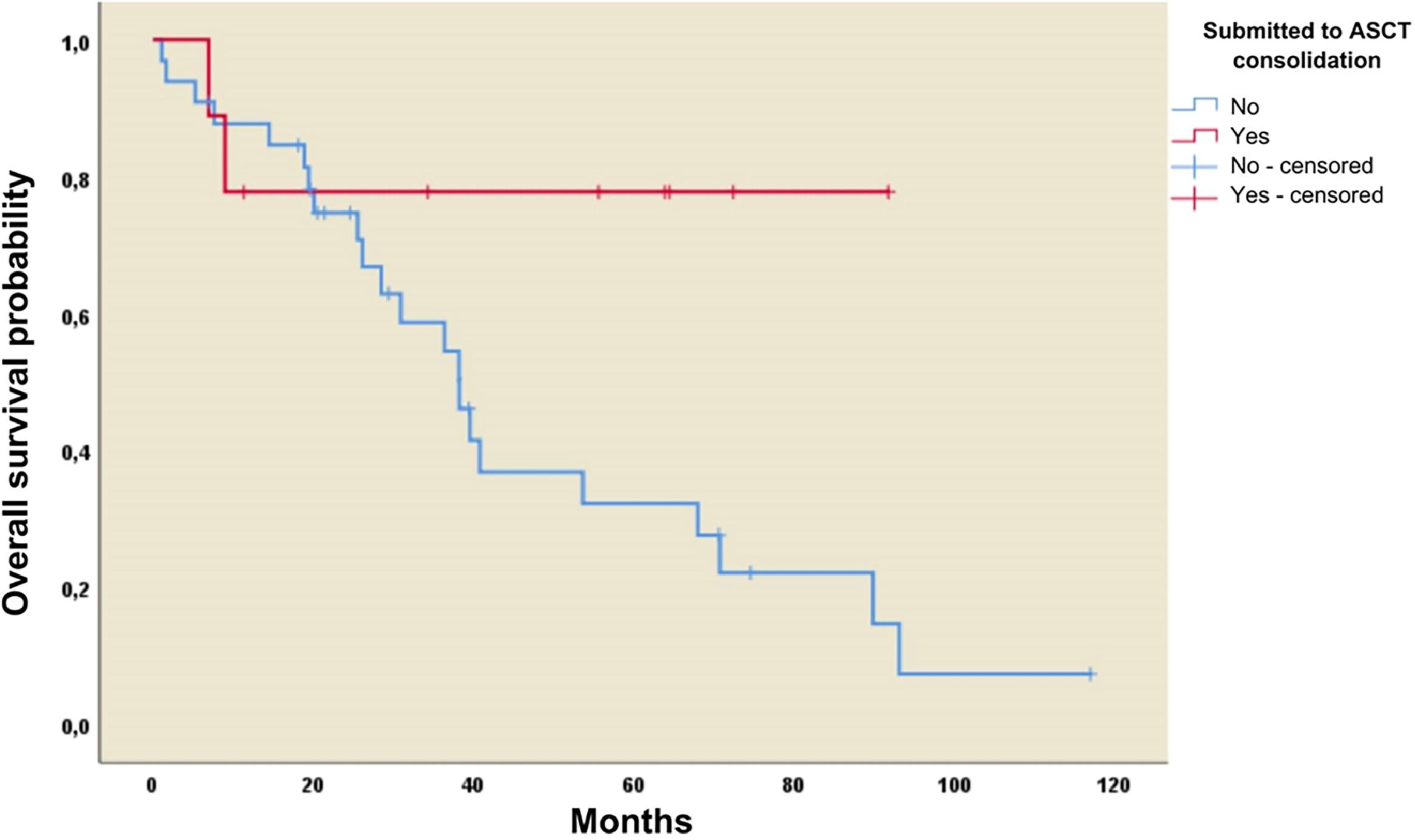
Three-year overall survival (OS) and progression-free survival (PFS) rates were 60 % and 44 %, respectively. Median OS and PFS were 3.41 (95 % CI: 1.6–5.22) and 2.98 (95 % CI: 2.5–3.46) years, respectively. There was no difference in median OS or PFS in patients receiving high-dose cytarabine and those that did not: 40.67 (95 % CI: 14.62–66.87) versus 39.54 (95 % CI: 2.41–76.66) months (p-value = 0.92) and 31.93 (95 % CI: 0.95–62.9) versus 34.92 (95 % CI 24.91–44.9) months (p-value = 0.85), respectively. The three-year OS rate was 78 % in patients who underwent ASCT versus 59 % in those not submitted to ASCT. Thus, the median OS was 38.2 months (95 % CI: 28.6–47.9; p-value = 0.06) in the group not receiving ASCT compared to the group receiving ASCT the mean was not reached (Figure 1a).
A statistically significant difference was observed in survival favoring patients with OR after first-line treatment compared to patients with stable disease or progression. More specifically, the median OS for patients with OR was 67.9 (95 % CI: 40.6–95.3) months versus 19.4 (95 % CI: 18.0–20.8) months (p-value ≤ 0.01) in patients with stable disease or progression. In the OR group, RM significantly improved median PFS: 38.2 (95 % CI: 32.39–44) compared to 31.4 (95 % CI: 9.5–53.2) months (p-value = 0.039; Figure 1b).
There were 22 R/R MCL patients. Three-year OS and PFS rates after R/R were 29 % and 11 %, respectively, with corresponding OS and PFS of 23.2 (95 % CI: 8.4–38) and 6.9 (95 % CI: 3.2–10.6) months. Ten patients (45.4 %) presented early progressive disease within 24 months (POD24). The cytarabine regimen was this groups most frequently used therapy (n = 6; 27.2 %), while three patients (13.4 %) received bendamustine. CR was achieved in six (27.2 %) patients and allogeneic ASCT was performed in two patients from the R/R group.
The present study provides insights into the clinical profile, treatment type, rate of ASCT as a consolidation strategy and survival of MCL patients in the pre-BTK inhibitor era in a single real-world setting in Brazil. Our results revealed that only 56.2 % of the 16 candidates were submitted to ASCT consolidation. These findings were more interesting after the first results of Triangle Trial, a phase 3, 3-arm study.6 The standard of care in the Triangle Trial was chemotherapy with a cytarabine-based plus rituximab regimen followed by ASCT. The second arm was chemotherapy plus rituximab and ibrutinib, followed by ASCT and ibrutinib maintenance for two years. The third arm was chemotherapy plus rituximab and ibrutinib. Patients who received ibrutinib had similar or even better outcomes than the standard-of-care patients followed by the ASCT arm with a more favorable safety profile. The second generation of BTK inhibitors (acalabrutinib and zanubrutinib) is highly selective and has the lowest adverse effects, potentially opening up future treatment opportunities for MCL.
Although our findings are compelling, the fact that it is from a single private center means that it does not widely represent the MCL population in Brazil. We believe that the present study can be expanded to a multicenter MCL registry in Brazil.
Formatting of funding sources
This paper was supported by an unconditional grant from AstraZeneca.