Congenital F-VII deficiency is a rare bleeding disorder caused by mutations of the F7-gene located on chromosome 13 long-arm and is believed to be inherited in an autosomal-recessive mode.1 It is encountered in approximately 1:300,000-500,000, whereas, the heterozygous form can reach 1: 350 individuals.2 Factor VII-deficiency is also characterized by several phenotypes and patients may be asymptomatic or may suffer from severe bleeding involving the GI system or the CNS.3 The major concern for these patients is bleeding during or after surgery/delivery. Levels of F-VII vary based on the type of mutation. The International Society on Thrombosis and Haemostasis has reclassified FVII deficiency as follows: severe, FVII <10%, risk of spontaneous major bleeding; moderate, FVII 10%–20%, risk of mild spontaneous or triggered bleeding; mild, FVII 20%–50%, mostly asymptomatic disease.4 Nevertheless, despite this classification, FVII activity level does not always correlate with bleeding severity. Patients with similar F-VII activity levels were found to have different bleeding patterns.5 Pregnancy is known to be a hypercoagulable state, where F-VII levels, among other coagulation factors, are typically increased towards the end of pregnancy.6 Bleeding during pregnancy and delivery can be concerning due to increased fetal and maternal morbidity. Whether prophylaxis during labor improves maternal or fetal outcome remains unclear and no guidelines have been made so far. The only existing systematic review included all cases reported between year 1953 and 2011.7 We opted to complement the aforementioned study with analysis of 18 new cases with F-VII deficiency published beyond 2012 along with a new woman managed at our center (a total of 19 cases).
Case presentationThis was a case of a 24-year-old Lebanese lady, G2P0A1, known to have congenital F-VII deficiency. Four years earlier she was diagnosed with F-VII deficiency after consulting a hematologist for easy bruising. Her blood exams were significant for elevated international normalized ratio (INR) of 1.3 and F-VII level of 35% (normal range 60-150%). Two years later, she had one spontaneous abortion at 15 weeks’ gestation that was complicated by hemorrhage, requiring treatment with rfVIIa (1.2 mg) and blood transfusion. Following treatment, F-VII level was found to be 40%. Her family history was significant for a paternal aunt carrier of F-VII-deficiency. The patient did not report any history of bleeding following tooth extraction, heavy menstruation, epistaxis, hematuria, rectal bleeding, excessive gingival bleeding or formation of spontaneous or traumatic hematomas.
The woman was referred to our outpatient clinic (OPD) at 35+5 weeks’ gestation. Her antenatal course was uneventful. Blood exam showed an INR of 1.07 and prolonged PTT (control 32s and patient 59s). Obstetric ultrasound done at 37+5 weeks of gestation showed a macrosomic baby with an estimated fetal weight of 3.9 kg (at 95th percentile for gestational age) and normal amniotic fluid index. She presented at 38+1 weeks in labor with regular contractions. Her Bishop score was 6/15. No cervical changes were noted after 5 hours of regular contractions. After multidisciplinary discussions, decision was taken to proceed with primary cesarean delivery, given the presence of fetal macrosomia and the potential for multiple complications including perineal trauma and instrumental delivery. In view of a history of postpartum hemorrhage following previous surgical abortion, prophylaxis with 1.2 mg rfVIIa (20mcg/kg) was given intravenous-drip immediately prior to surgery. Cesarean was done under general anesthesia. Patient was delivered of a live born male infant weighing 3970 grams with optimal Apgar Score. Delivery was complicated by uterine atony with estimated blood loss of 800 cc. Bleeding was controlled with Pitocin IV (20UI), 1gr Tranexamic acid, 3 tablets of Misoprostol (200mcg) rectally. Hemovac-drain was placed intraoperatively. Patient did not require any transfusion of packed red blood cells nor fresh frozen plasma. rfVIIa was continued 1.2 mg intravenous drip every 12 hours for 7 days as recommended by hematology team. The newborn infant was transferred to the normal nursery; the circumcision and the intramuscular injection of vitamin K were deferred. No neonatal hemorrhage was noted. Postpartum course was uncomplicated, hemoglobin dropped from 12.6 to 10.9. Hemovac drain was removed on day 2 post-operatively. The puerperium progressed uneventfully and no adverse events after discontinuing the replacement therapy. The woman and her infant were found to be healthy on routine check-up-visit 6 weeks postpartum.
Discussion and conclusionsThis patient was a 24 year-old lady whose last F-VII activity level before delivery was 40% (mild F-VII-deficiency), yet, given a history of hemorrhage following previous abortion at 15 weeks’ gestation that responded to rfVIIa replacement therapy and blood transfusion, we elected to proceed with peripartum rfVIIa prophylaxis in current pregnancy. She received 1.2 milligrams intravenous drip immediately prior to her operation followed by similar doses every 12 hours for 7 days as recommended by the hematology team. This dose seemed to be sufficient in this case as no intrapartum bleeding was observed. The operative delivery was uneventful in spite of the development of transient uterine atony that was successfully managed with massaging and uterotonics. The uterine bleeding immediately accompanied uterine atony. This happened even after receiving rfVIIa 30 minutes before delivery which has certainly raised F-VII activity to levels > 40%. No bleeding was observed from surgical sites. Usually, when F-VII activity level is below 10 IU/dL, clinically apparent bleeding diathesis emerges8 and it is generally agreed that supplementation with rfVIIa in these patients is a reasonable approach before surgery/delivery9 and this is usually administered 30-60 min prior to surgery.10 Nevertheless, for unclear reasons, individuals with milder forms can also manifest bleeding especially in association with surgery/delivery rendering correlation between F-VII activity levels and bleeding tendency very poor. Replacement for patients with higher F-VII activity levels, however, remains controversial. Management and delivery of women with milder F-VII deficiency is mostly approached on a case-by-case basis.11 Beneficial effect of replacement before delivery to all patients lacks scientific evidence and its use could to be associated with risks of thrombosis and the development of inhibitory antibodies12 in addition to being expensive and not always readily available. A longitudinal study of 100 women with uneventful pregnancies showed a progressive rise in F-VII coagulant activity and F-VII antigen throughout pregnancy. These observations were concluded from apparently healthy women with no deficiencies in any of the coagulation factors. This increase in F-VII activity was also noted in mild-moderate deficiency.13 Kulkarni et al in 2006, also suggested that the risk of bleeding is increased in the first trimester of pregnancy when compared to later pregnancy due to inadequate increase in F-VII activity level in early pregnancy.6 This could explain why hemorrhage in this patient was encountered in previous first trimester abortion and not during current delivery. The decision regarding replacement therapy during labor and delivery should be individualized, depending on mother's bleeding tendency, FVII level in third trimester and the mode of delivery.6 Yet, others did not find individuals’ bleeding history to predict the need for replacement before delivery.7
We performed a literature review and comprehensive search of the medical databases using the terms “F-VII deficiency” and “pregnancy”. We limited our search to articles published between 2012-2021. This yielded articles from PubMed (n = 31), MEDLINE (n = 12), Embase (n = 78), CINAHL (n = 5) and Cochrane library (n = 0). Search was limited to inherited or congenital FVII deficiency. Abstracts from 126 articles were reviewed after exclusion of duplicates. References from relevant articles were also assessed. We excluded articles involving combined coagulopathies or when FVII deficiency presented as a component of a syndrome. We also excluded articles and abstracts that did not discuss intrapartum management of Factor VII deficiency. Only English and French articles were considered.
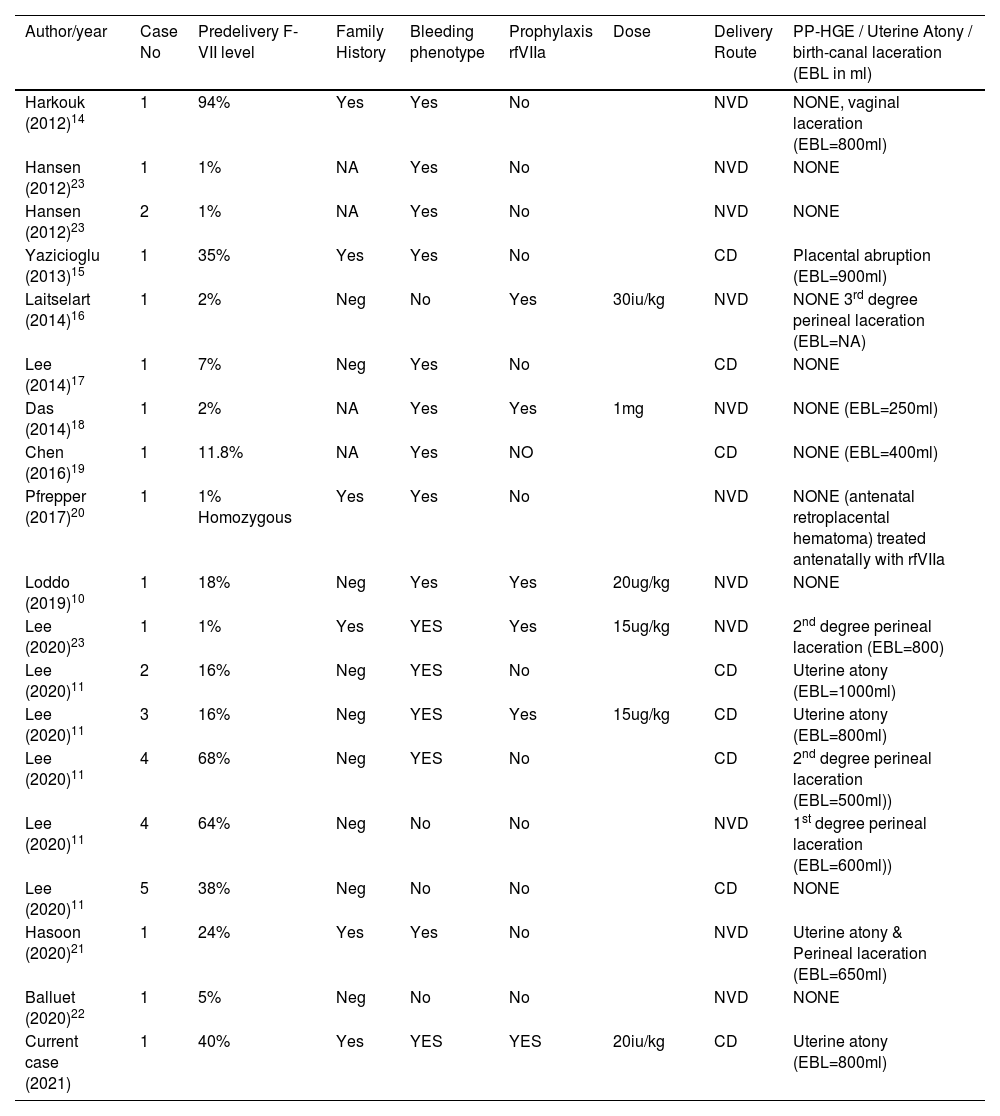
Identified, were ten relevant articles reporting on 10 cases,10,14-22 another one reporting 5 cases with 6 pregnancies and last one describing two cases.23 A new case managed at our center was added to this series (totaled 19 cases). A summary of prominent features was displayed in Table 1.
Recent similar cases reported to the literature with relevant clinical findings.
| Author/year | Case No | Predelivery F-VII level | Family History | Bleeding phenotype | Prophylaxis rfVIIa | Dose | Delivery Route | PP-HGE / Uterine Atony / birth-canal laceration (EBL in ml) |
|---|---|---|---|---|---|---|---|---|
| Harkouk (2012)14 | 1 | 94% | Yes | Yes | No | NVD | NONE, vaginal laceration (EBL=800ml) | |
| Hansen (2012)23 | 1 | 1% | NA | Yes | No | NVD | NONE | |
| Hansen (2012)23 | 2 | 1% | NA | Yes | No | NVD | NONE | |
| Yazicioglu (2013)15 | 1 | 35% | Yes | Yes | No | CD | Placental abruption (EBL=900ml) | |
| Laitselart (2014)16 | 1 | 2% | Neg | No | Yes | 30iu/kg | NVD | NONE 3rd degree perineal laceration (EBL=NA) |
| Lee (2014)17 | 1 | 7% | Neg | Yes | No | CD | NONE | |
| Das (2014)18 | 1 | 2% | NA | Yes | Yes | 1mg | NVD | NONE (EBL=250ml) |
| Chen (2016)19 | 1 | 11.8% | NA | Yes | NO | CD | NONE (EBL=400ml) | |
| Pfrepper (2017)20 | 1 | 1% Homozygous | Yes | Yes | No | NVD | NONE (antenatal retroplacental hematoma) treated antenatally with rfVIIa | |
| Loddo (2019)10 | 1 | 18% | Neg | Yes | Yes | 20ug/kg | NVD | NONE |
| Lee (2020)23 | 1 | 1% | Yes | YES | Yes | 15ug/kg | NVD | 2nd degree perineal laceration (EBL=800) |
| Lee (2020)11 | 2 | 16% | Neg | YES | No | CD | Uterine atony (EBL=1000ml) | |
| Lee (2020)11 | 3 | 16% | Neg | YES | Yes | 15ug/kg | CD | Uterine atony (EBL=800ml) |
| Lee (2020)11 | 4 | 68% | Neg | YES | No | CD | 2nd degree perineal laceration (EBL=500ml)) | |
| Lee (2020)11 | 4 | 64% | Neg | No | No | NVD | 1st degree perineal laceration (EBL=600ml)) | |
| Lee (2020)11 | 5 | 38% | Neg | No | No | CD | NONE | |
| Hasoon (2020)21 | 1 | 24% | Yes | Yes | No | NVD | Uterine atony & Perineal laceration (EBL=650ml) | |
| Balluet (2020)22 | 1 | 5% | Neg | No | No | NVD | NONE | |
| Current case (2021) | 1 | 40% | Yes | YES | YES | 20iu/kg | CD | Uterine atony (EBL=800ml) |
EBL: estimated blood loss; Nl: normal; Cs: C-section; NVD: normal vaginal delivery; PPH: post-partum hemorrhage; NA: not available.
One woman carried twin gestation11 while remaining women had singleton pregnancies. All women gave birth at full-term except one case who was delivered by cesarean section at 29 weeks’ gestation due to placental abruption and associated fetal distress.15 Of these 19 women, 11 deliveries were by vaginal route and 8 were by cesarean sections. Median maternal age was 27 years (17-39) and were predominantly multigravidas (12/19). Family history was available in 14/19, positive in 6/14 and negative in 8/14. Bleeding phenotype was positive in 15/19 cases. Of these 15, 7 had a history of antecedent obstetric bleeding particularly accompanying abortions and 5 recalled successful treatment with rfVIIa. Severe disease (F-VII <10%) was documented in 8 cases while 11 cases had levels ranging between 11.8 – 94% (median of 35%). Factor VII replacement (rfVIIa) was administered before 4 vaginal and 2 cesarean deliveries. Of these 6 cases, three had severe disease while the remaining 3 had the mild-moderate form. On the other hand, the presence of a history of previous bleeding tendency (bleeding-phenotype) was present in 5/6 cases receiving replacement prophylaxis. Intrapartum or postpartum hemorrhage was not encountered in any delivery. Nevertheless, we could observe excessive bleeding related to the development of associated obstetric complications. Four developed uterine atony, all of whom in the mild-moderate disease category, 1 placental abruption in a woman with mild-moderate disease, 1 antenatal retroplacental hematoma in a woman with severe disease and 6 cases of birth-canal lacerations where 4 cases were with mild-moderate and two were with severe disease. These women were treated adequately with uterotonics, received transfusions with blood/FFP but none required treatment with rfVIIa. In their review, Kreuziger et al reported that women with FVII-deficiency who underwent cesarean section were 3 times more likely to receive prophylaxis with rfVIIa.7 However, the authors concluded that rfVIIa prophylaxis should not be mandatory as it did not decrease the risk of postpartum hemorrhage.7 This association was not observed in this group, where FVII level did not influence the decision to administer prophylaxis nor it influenced the route of delivery. In the above mentioned review, postpartum hemorrhage complicated 54% of women who did not received prophylaxis with rfVIIa as opposed to only 22% of women who received prophylaxis.7 Few case reports on factor VII deficiency in pregnancy exist and there is no consensus or clear guidelines regarding optimal management of pregnant women with mild-moderate F-VII deficiency.6 rfVIIa is a recombinant F-VII with half-life of 4 to 5 hours. Although a protocol for management of acute bleeding exists, there is no protocol for prophylaxis prior to delivery.7 Loddo et al. compiled a list of different prophylactic protocols described in previous case reports with doses ranging from 13.3 microgram/kg body weight to 60 microgram/kg at delivery followed by additional doses after delivery.10 Factor VII deficiency is the most common of the rare Inherited Coagulation Disorders. It is known that the bleeding phenotype does not correlate with the factor VII level. Several studies have shown that history of bleeding is the most reliable criteria to predict the risk of bleeding during surgery, thereby, permitting proper selection of patients who may benefit from prophylaxis.24 Patients with abundant epistaxis leading to iron deficiency and hospitalization or with soft tissue hematomas might benefit from F-VII prophylaxis before surgeries (including normal vaginal deliveries and cesarean deliveries) in order to reduce the risk of bleeding.24 This was also observed among cases in our review, where 5/6 women who received prophylaxis belonged to patients with bleeding phenotypes.
To conclude, replacement with rfVIIa before delivery is a rational approach for women with severe disease. In view of the absence of a guideline for women with milder forms of the disease, care should be taken to consider F-VII activity level together with the clinical presentation and history of bleeding before deciding to implement rfVIIa prophylaxis. Delivery of these women, whether by cesarean or vaginal route, should better take place at centers with immediate accessibility to blood products and rfVIIa. Care can optimally be provided by a multidisciplinary team including a hematologists, anesthetists and maternal-fetal obstetricians.
Authors' contributionsRH and AK collected the data. IH and MKR analyzed data in view of the literature review, elaborated and wrote the paper. RJ and RC revised the paper. The authors read and approved the final manuscript.