Immune dysfunction and thrombocytopenia are common features in liver cirrhosis. Platelet transfusion is the most widely used therapeutic approach for thrombocytopenia when indicated. The transfused platelets are prone to lesions during their storage that empower their interaction with the recipient leucocyte. These interactions modulate the host immune response. The impact of platelet transfusion on the immune system in cirrhotic patients is little understood. Therefore, this study aims to investigate the impact of platelet transfusion on neutrophil function in cirrhotic patients.
MethodsThis prospective cohort study was implemented on 30 cirrhotic patients receiving platelet transfusion and 30 healthy individuals as a control group. EDTA blood samples were collected from cirrhotic patients before and after an elective platelet transfusion. Flowcytometric analysis of neutrophil functions (CD11b expression and PCN formation) was performed.
ResultsThere was a significant increase in expression of CD11b on neutrophils and Frequency of platelet-complexed neutrophils (PCN) in patients with cirrhosis compared with controls. Platelet transfusion increased level of CD11b and the frequency of PCN even more. There was a significant positive correlation between change in PCN Frequency pefore and after transfusion and the change in expression of CD11b among cirrhotic patients.
ConclusionsElective platelet transfusion appears to increase level of PCN in cirrhotic patients, moreover, exacerbate the expression of activation marker CD11b on both neutrophils and PCN. More research and studies are needed to corroborate our preliminary findings.
Immune dysfunction has been introduced as a key player in the evolution of liver cirrhosis that starts with chronic hepatitis and worsens with the progression to cirrhosis and hypertension. This dysfunction mainly affects the innate immune system.1
Patients suffering from liver cirrhosis usually suffer from thrombocytopenia. Several mechanisms may be involved in the development of thrombocytopenia in liver diseases, including the decreased thrombopoietin (TPO) production by the liver and sequestration of platelets in the enlarged spleen.2 Platelet transfusion and TPO receptor agonists are the two alternative strategies used to increase the platelet count, when indicated.3
In addition to the well-known hemostatic function, platelets also have immunomodulatory functions. These functions are mediated either by secreted molecules (cytokines and chemokines) or surface molecules involved in the interaction between platelets and immune cells. The transfused platelets acquire storage lesions, during storage in blood banks and these lesions play a part in inducing the expression of these molecules, even in the absence of other stimuli.4
The interaction between the platelet and the immune system is very complex. Some recent studies suggest that platelets, in addition to their pro-inflammatory effect, may have an anti-inflammatory effect in some conditions.5
The divergent immunomodulatory effect of platelets attracted much attention due to their underlying therapeutic potential. Some studied claimed that the use of platelets, after abolishing the hemostatic properties, can be promising in early stages of sepsis due to its pro-inflammatory properties.6 Others postulate that platelets be used to alleviate inflammatory conditions, using their anti-inflammatory mechanisms.5
Despite many researchers having investigated the immune-mediated effect of platelets, the effect of interactions between platelets and immune cells on the host immune system remains to be clarified.7
Here, we aimed to investigate the interaction between transfused platelets and neutrophils, as innate immune cells, in patients with cirrhosis.
Subjects and methodsDesignThis prospective cohort study was conducted on 30 patients with cirrhosis due to chronic hepatitis C virus (HCV) infection who were planned to receive prophylactic elective platelet transfusions to prevent hemorrhage prior to invasive procedures; platelets were transfused when the platelet count in non-bleeding patients was less than 50, 000/µL. Platelet transfusions were ABO-matched and consisted of pooled platelets obtained by centrifuging 6 units of whole blood within 4 to 6 hours of collection, referred to as random donor platelets (RDP). One unit is expected to increase the platelet count by 5,000 to 10,000/µL in a non-refractory patient. The usual adult dose was approximately 4 - 6 units of pooled random donor platelets, depending on the baseline platelet count. Blood samples were taken prior to the administration of platelets and repeated one hour after transfusion. Furthermore, another 30 age and gender-matched healthy volunteers were included. The patients were recruited from the interventional radiology department at the National Liver Institute of Menoufia University. All subjects gave informed consent approved by the ethical scientific committee of the National Liver Institute before participating in the study.
Inclusion criteriaPatients between the ages of 18 and 75 who had been diagnosed with cirrhosis by ultrasonography or histopathology and were scheduled for an elective platelet transfusion were enrolled. There were no hepatic or thrombotic disorders in the healthy control group.
Exclusion criteriaPatients who had received a platelet transfusion in the previous two weeks, received plasma prior to, or along with, platelet transfusion, been treated with any drugs that affect leucocyte or platelet function, such as anti-platelet therapies, anti-coagulants or immunomodulatory drugs, had evidence of acute infection and undergone liver transplantation were excluded.
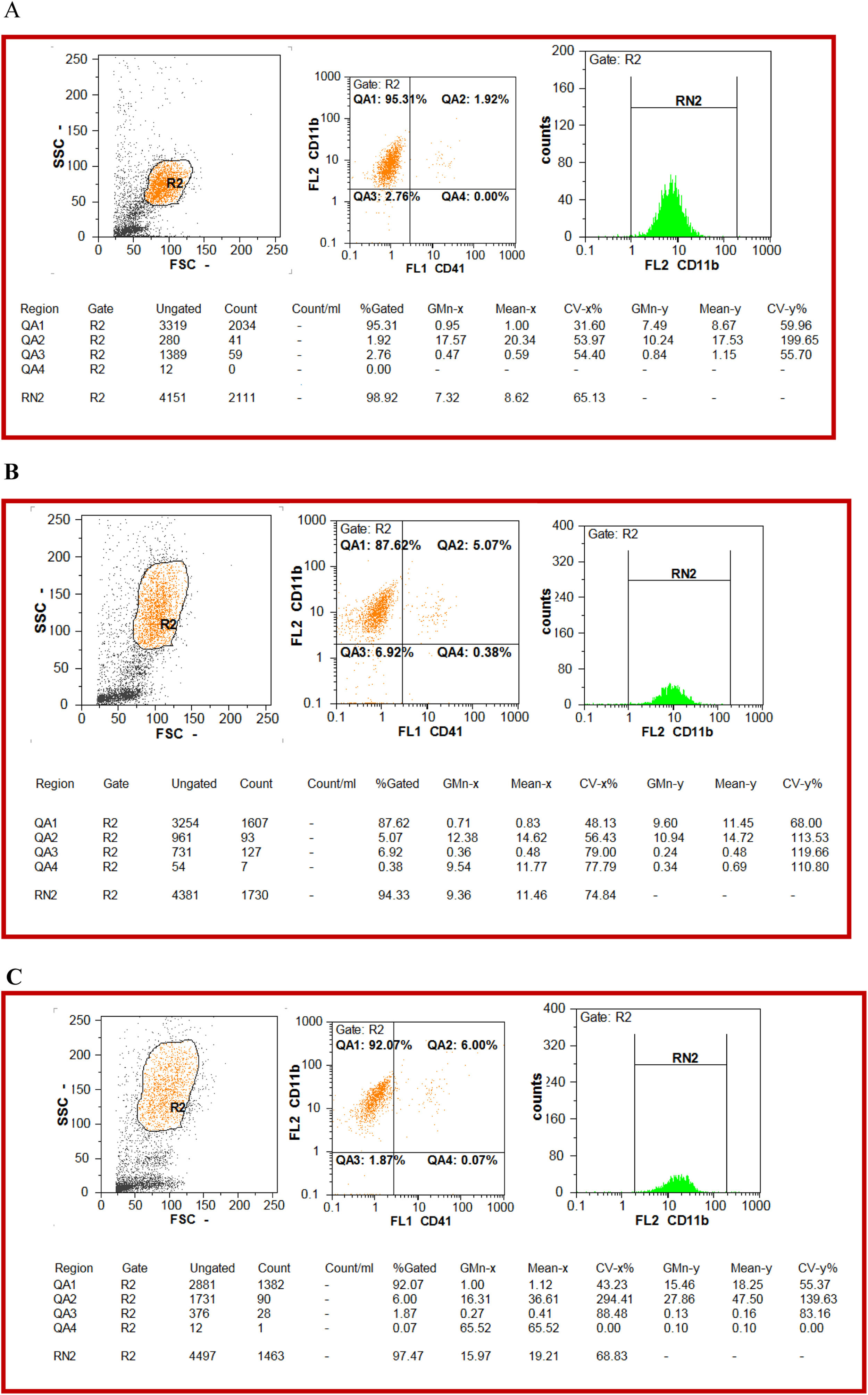
MethodsTwo milliliters of venous blood were withdrawn from every subject under complete aseptic conditions and transferred to tubes containing K2EDTA. Pre- and post-transfusion samples were obtained from patients with liver cirrhosis. The samples were subjected to surface staining with optimized amounts of fluorochrome-conjugated antibodies, CD41a-FITC, CD11b-PE. 100 µl of anticoagulated (EDTA) whole blood was added to the bottom of a 12 × 75 mm. polystyrene tube, along with 5µl of CD11b in one tube, with 5µl of CD11b and 5µl of CD41 in another tube, and vortex and incubation were performed for 40 minutes in the dark at room temperature. Following the staining process, lysis of RBCs was performed with the lysis solution (Dako Uti-Lyse™), consisting of two reagents. Reagent A lysed red blood cells in whole blood samples appropriately to perform monoclonal antibody panel analysis without interference from red blood cells. Reagent B slowed the lysing reaction to allow analysis without damage to white blood cells. The volume of 100 µl of reagent A was added to the sample, vortex, and incubation were performed for 10 minutes at room temperature in the dark. The volume of 1 mL of reagent B was added to the sample, vortex and incubation were performed for 10 minutes at room temperature in the dark. Unstained samples were included as controls. The flowcytometric analysis was performed using (Partec CyFlow® Space Flow Cytometer). The gating of neutrophils was performed by forward and side scatter. The mean fluorescence intensity (MFI) of the CD11b expression was used to assess the activation of neutrophils. The frequency of platelet-complexed neutrophils (PCNs) was measured by the co-expression of the platelet marker CD41a (platelet glycoprotein alpha-IIb) and the neutrophil marker CD11b (Figure 1).
Flow cytometry represents approach to quantify circulating platelet-complexed neutrophils (PCNs) and CD11b on neutrophils. Neutrophil (R2) populations are defined by their forward and side scatter characteristics, PCN are quantified as the percentage of neutrophils (R2) that stain for CD11b and CD41[QA2], Mean fluorescence intensity (MFI) of CD11b was measured on neutrophils (R2) [RN2] A) A representative of healthy person, the percentage of PCN [QA2 = 1.92%] [MFI = 10.24], Mean fluorescence intensity (MFI) of CD11b on neutrophils (R2) [RN2 = 98.9%] [MFI = 7.32]. B) A representative of cirrhotic patient before platelet transfusion, the percentage of PCNs [QA2 = 5.07%] [MFI = 10.94], mean fluorescence intensity (MFI) of CD11b on neutrophils (R2) [RN2 = 94.33%] [MFI = 9.36]. C) A representative of cirrhotic patient after platelet transfusion, the percentage of PCNs [QA2 = 6.00] [MFI = 27.86], MFI of CD11b on neutrophils (R2) [RN2 = 97.47%] [MFI = 15.97].
The data were analyzed using the Statistical Package of Social Sciences (SPSS 22.0, IBM/SPSS Inc., Chicago, IL). There were two types of statistical analysis performed. The quantitative variables were compared using the Student's t, Shapiro-Wilk and Mann-Whitney tests. The qualitative variables were compared using the Chi-square (2) and Fisher's Exact tests. The paired t-test was used to test for significant differences between pairs of values (change from before to after transfusion). The Wilcoxon signed-rank test (non-parametric), equivalent for the paired t-test, was used when the normality assumption was violated. The Spearman rank correlation coefficient (rs) was used for the correlation study. All these tests were used as tests of significance at p < 0.05.
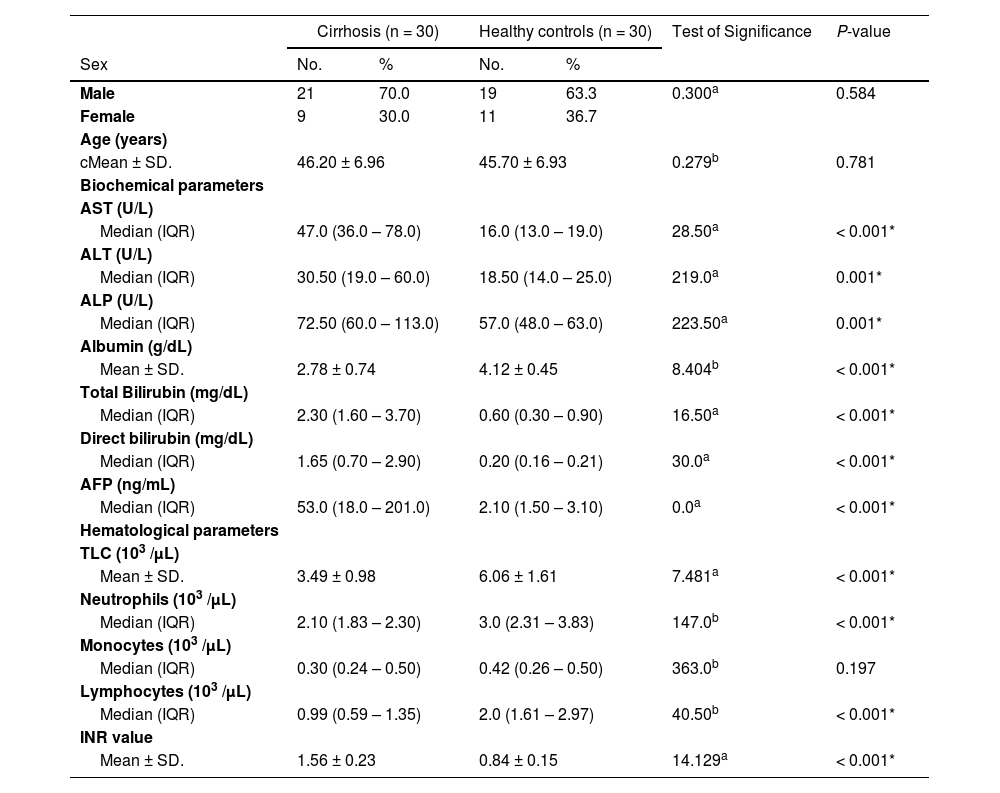
ResultsThis study enrolled 30 patients with liver cirrhosis and another 30 age and gender-matched healthy volunteers. The baseline characteristics of healthy controls and patients with liver cirrhosis before platelet transfusion are illustrated in Table 1.
Baseline characteristics of healthy controls and patients with liver cirrhosis before platelet transfusion.
| Cirrhosis (n = 30) | Healthy controls (n = 30) | Test of Significance | P-value | |||
|---|---|---|---|---|---|---|
| Sex | No. | % | No. | % | ||
| Male | 21 | 70.0 | 19 | 63.3 | 0.300a | 0.584 |
| Female | 9 | 30.0 | 11 | 36.7 | ||
| Age (years) | ||||||
| cMean ± SD. | 46.20 ± 6.96 | 45.70 ± 6.93 | 0.279b | 0.781 | ||
| Biochemical parameters | ||||||
| AST (U/L) | ||||||
| Median (IQR) | 47.0 (36.0 – 78.0) | 16.0 (13.0 – 19.0) | 28.50a | < 0.001* | ||
| ALT (U/L) | ||||||
| Median (IQR) | 30.50 (19.0 – 60.0) | 18.50 (14.0 – 25.0) | 219.0a | 0.001* | ||
| ALP (U/L) | ||||||
| Median (IQR) | 72.50 (60.0 – 113.0) | 57.0 (48.0 – 63.0) | 223.50a | 0.001* | ||
| Albumin (g/dL) | ||||||
| Mean ± SD. | 2.78 ± 0.74 | 4.12 ± 0.45 | 8.404b | < 0.001* | ||
| Total Bilirubin (mg/dL) | ||||||
| Median (IQR) | 2.30 (1.60 – 3.70) | 0.60 (0.30 – 0.90) | 16.50a | < 0.001* | ||
| Direct bilirubin (mg/dL) | ||||||
| Median (IQR) | 1.65 (0.70 – 2.90) | 0.20 (0.16 – 0.21) | 30.0a | < 0.001* | ||
| AFP (ng/mL) | ||||||
| Median (IQR) | 53.0 (18.0 – 201.0) | 2.10 (1.50 – 3.10) | 0.0a | < 0.001* | ||
| Hematological parameters | ||||||
| TLC (103 /μL) | ||||||
| Mean ± SD. | 3.49 ± 0.98 | 6.06 ± 1.61 | 7.481a | < 0.001* | ||
| Neutrophils (103 /μL) | ||||||
| Median (IQR) | 2.10 (1.83 – 2.30) | 3.0 (2.31 – 3.83) | 147.0b | < 0.001* | ||
| Monocytes (103 /μL) | ||||||
| Median (IQR) | 0.30 (0.24 – 0.50) | 0.42 (0.26 – 0.50) | 363.0b | 0.197 | ||
| Lymphocytes (103 /μL) | ||||||
| Median (IQR) | 0.99 (0.59 – 1.35) | 2.0 (1.61 – 2.97) | 40.50b | < 0.001* | ||
| INR value | ||||||
| Mean ± SD. | 1.56 ± 0.23 | 0.84 ± 0.15 | 14.129a | < 0.001* | ||
IQR: Interquartile range.
The MFI of the CD11b expression on neutrophils was higher in the pre-transfused cirrhotic patients than in the controls. Furthermore, the frequency of PCNs was increased in pre-transfused cirrhotic patients, compared to the controls (Table 2).
The MFI of CD11b on neutrophils and percentage of platelet complexed neutrophils (PCNs) were compared between the pre-transfused cirrhotic patients and healthy controls.
| Pre-transfused Cirrhotic patients (n = 30) | Healthy controls (n = 30) | Mann Whitney test | P-value | |
|---|---|---|---|---|
| MFI of CD11b on Neutrophils | ||||
| Median (IQR) | 16.84 (11.88 – 19.78) | 6.89 (4.70 – 13.40) | 183.0 | < 0.001* |
| PCN % | ||||
| Median (IQR) | 3.93 (3.10 – 5.0) | 1.70 (1.06 – 7.20) | 261.0 | 0.005* |
IQR: Interquartile range.
MFI: Mean fluorescence intensity.
PCN: platelet complexed neutrophils.
There was a highly significant statistical increase in the MFI of the CD11b expression on neutrophils after platelet transfusion in cirrhotic patients. The frequency of PCNs was significantly increased in post-transfusion samples, compared to the pre-transfusion samples (Table 3).
The MFI of CD11b on neutrophils and percentage of platelet-complexed neutrophils (PCNs) were compared in the cirrhosis group, before and after platelet transfusion.
| Cirrhosis pre-platelet transfusion (n = 30) | Cirrhosis post-platelet transfusion (n = 30) | Wilcoxon signed ranks test | P-value | |
|---|---|---|---|---|
| MFI of CD11b on Neutrophils | ||||
| Median (IQR) | 16.84 (11.88 – 19.78) | 23.23 (17.49 – 32.60) | 4.783 | < 0.001* |
| PCN % | ||||
| Median (IQR) | 3.93 (3.10 – 5.0) | 9.50 (6.99 – 18.0) | 4.783 | < 0.001* |
IQR: Interquartile range.
MFI: Mean fluorescence intensity.
PCN: platelet complexed neutrophils.
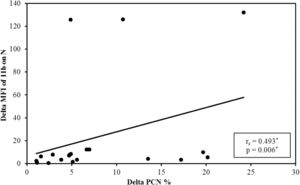
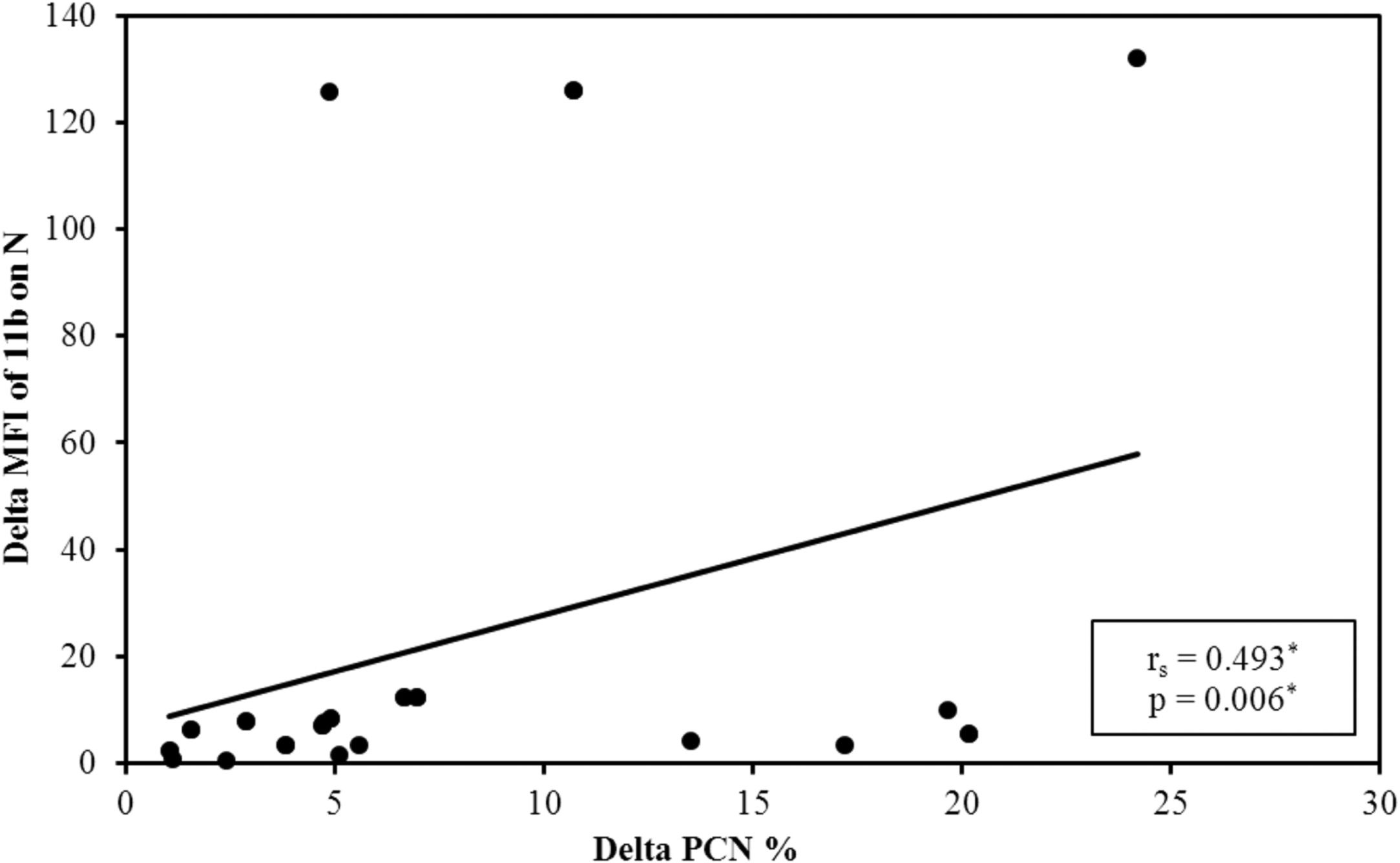
It was observed that after platelet transfusion, the change in the PCN% was positively correlated with increasing levels of platelets and the MFI of the CD11b on neutrophils, compared to their values before platelet transfusion (Figure 2).
DiscussionDuring the course of liver cirrhosis, neutrophils, as the largest population of innate immune cells, show an alteration in function that may be involved in the worsening of the disease condition.1
In this study, we evaluated the change in neutrophil functions mediated by platelet transfusion in patients with liver cirrhosis.
In the current study, patients with liver cirrhosis showed increased baseline expression of the CD11b on neutrophils. The increased expression of the CD11b was also reported by Huang et al.8
It is to be mentioned that the CD11b is expressed on the cell surface as a part of the heterodimer (CD11b/CD18), which is one of the β2 integrins that are not only involved in neutrophil adhesion and recruitment, but also, in other neutrophil effector functions, such as phagocytosis and intracellular killing. The CD11b/Cd18 is also named Mac-1 and acts as the complement receptor 3.9
In spite of the paucity in studies regarding the alterations in platelet functions in liver cirrhosis, some studies reported that platelets show increased level of p-selectin expression and increased the platelet – leucocyte aggregate formation.10
The present study also revealed an increased PCN formation in patients with liver cirrhosis. This is in agreement with the data reported by Støy et al.11
The formation of the PCN is mediated by the interactions between many adhesion molecules, mainly the p-selectin on the platelet surface and the p-selectin glycoprotein ligand-1 on the leucocyte surface.12
The platelet neutrophil aggregates formation facilitates their recruitment and their extravascular movement. Targeting these aggregates may diminish the neutrophil migration in experimental studies.13
Deppermann et al.12 claimed that the PCN formation affect neutrophil functions, including the neutrophil adhesiveness and the formation of the neutrophil extracellular trap and reactive oxygen species.
The interaction between platelet and both innate and adaptive immune cells has gained much interest, especially in the perspective of platelet transfusion. It has been suggested that platelets influence neutrophil recruitment and neutrophil extracellular trap formation.14 It has also been assumed that platelets influence monocyte polarization toward M1 pro-inflammatory phenotype.6 On the contrary, it has been proposed that platelets inhibit the proinflammatory function of dendritic cells, thus inhibiting the T cell priming.
This study demonstrated that platelet transfusion increased the expression of the CD11b and the PCN formation. Moreover, there was a positive correlation between the change in PCN and the change in the CD11b expression. 11.Støy et al.,11 reported the same positive correlation and thus, assumed that the CD11b plays role in the PCN formation. This could be mediated by an interaction between the CD11b and PGIb. However, 11.Støy and his colleagues declared a significant increase in the platelet-complexed monocyte, but not in the PCN, after the platelet transfusion. Furthermore, it need be mentioned that 5. Hally et al., postulated that the co-culture of autologous platelets with neutrophils showed a decreased, rather than increased, CD11b expression in vitro. 16.Stocker et al.,14 assumed that the platelet can reduce the CD11b expression on neutrophils through their GPIb.
The inconsistency in the findings raises the question about factors that determine the variable immunomodulatory effect of platelet transfusion. Platelets can induce either pro-inflammatory or anti-inflammatory effects. The choice between the two divergent phenotypes may depend, at least in part, on ligation of certain receptors on platelets. The specific phenotype may be determined at early stages of megakaryocytes and this predetermined phenotype can also be altered later on by different factors.6
Further studies about the overall immunomodulatory effects of platelet transfusion and whether the immunomodulatory effects of platelet transfusion are beneficial or hazardous in the context of liver cirrhosis, with regard to the stage, presence of complications or concomitant conditions, could be very beneficial. This could help in determining in which conditions the transfusion of platelets could be beneficial in those patients and when to shift to other therapeutic alternatives.
ConclusionThis study concluded that, in the perspective of liver cirrhosis, platelet transfusion possesses a proinflammatory effect regarding neutrophil functions.




![Flow cytometry represents approach to quantify circulating platelet-complexed neutrophils (PCNs) and CD11b on neutrophils. Neutrophil (R2) populations are defined by their forward and side scatter characteristics, PCN are quantified as the percentage of neutrophils (R2) that stain for CD11b and CD41[QA2], Mean fluorescence intensity (MFI) of CD11b was measured on neutrophils (R2) [RN2] A) A representative of healthy person, the percentage of PCN [QA2 = 1.92%] [MFI = 10.24], Mean fluorescence intensity (MFI) of CD11b on neutrophils (R2) [RN2 = 98.9%] [MFI = 7.32]. B) A representative of cirrhotic patient before platelet transfusion, the percentage of PCNs [QA2 = 5.07%] [MFI = 10.94], mean fluorescence intensity (MFI) of CD11b on neutrophils (R2) [RN2 = 94.33%] [MFI = 9.36]. C) A representative of cirrhotic patient after platelet transfusion, the percentage of PCNs [QA2 = 6.00] [MFI = 27.86], MFI of CD11b on neutrophils (R2) [RN2 = 97.47%] [MFI = 15.97]. Flow cytometry represents approach to quantify circulating platelet-complexed neutrophils (PCNs) and CD11b on neutrophils. Neutrophil (R2) populations are defined by their forward and side scatter characteristics, PCN are quantified as the percentage of neutrophils (R2) that stain for CD11b and CD41[QA2], Mean fluorescence intensity (MFI) of CD11b was measured on neutrophils (R2) [RN2] A) A representative of healthy person, the percentage of PCN [QA2 = 1.92%] [MFI = 10.24], Mean fluorescence intensity (MFI) of CD11b on neutrophils (R2) [RN2 = 98.9%] [MFI = 7.32]. B) A representative of cirrhotic patient before platelet transfusion, the percentage of PCNs [QA2 = 5.07%] [MFI = 10.94], mean fluorescence intensity (MFI) of CD11b on neutrophils (R2) [RN2 = 94.33%] [MFI = 9.36]. C) A representative of cirrhotic patient after platelet transfusion, the percentage of PCNs [QA2 = 6.00] [MFI = 27.86], MFI of CD11b on neutrophils (R2) [RN2 = 97.47%] [MFI = 15.97].](https://static.elsevier.es/multimedia/25311379/0000004500000004/v1_202310231039/S2531137922014249/v1_202310231039/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w93OM6WmS6o9DeZl+SVh74uo=)