Thrombotic thrombocytopenic purpura (TTP) is a potentially fatal disease that requires early diagnosis and treatment that can be made possible by applying the PLASMIC score. This study aims to evaluate this score applicability for patients with suspected TTP in a developing country.
MethodsThis was a retrospective study performed at a tertiary hospital in the northeastern region of Brazil. Patients were analyzed in two groups: ADAMTS13 activity <10% and activity >10%. Patients were stratified according to the PLASMIC score, and the level of agreement between the PLASMIC score and the ADAMTS13 activity was evaluated.
ResultsEight patients with thrombotic microangiopathy were included. Four patients had ADAMTS13 activity <10%, all with a PLASMIC score =6. The other four had ADAMTS13 activity >10%, all with a score <6. Based on a score =6 for presumptive diagnosis of TTP, we attained a 100% diagnostic accuracy in our sample. The PLASMIC score was also able to accurately predict response to plasma exchange and the risk of long-term unfavorable outcomes.
ConclusionsThe reproducibility of the PLASMIC score was quite satisfactory in our sample. It accurately discriminates between patients who had ADAMTS13 deficiency and those with normal enzyme activity, precluding the need for specific laboratory evaluation, which is not always available. This score can be useful for an early diagnosis and indicates which patients will benefit from the treatment in developing countries.
Initially described in 1924 by Eli Moschcowitz1 in a 16-year-old patient, thrombotic thrombocytopenic purpura (TTP) is a disorder characterized by microangiopathic hemolytic anemia, thrombocytopenic purpura and neurologic abnormalities. In 1997, Furlan et al.,2 concomitant to Tsai,3,4 described that the von Willebrand (vWF) cleaving protein – ADAMTS13 (a disentegrin and metalloproteinase with thrombospondin type 1 motif 13) – was deficient in patients with TTP.2–9
The presence of anti-ADAMTS13 IgG-class antibodies was later shown in cases of acquired disease.3,4,10–15 The existence of these antibodies is the rationale to the first-line treatment of TTP with plasma exchange and corticosteroids.4,6,16–20 This kind of treatment has improved the prognosis: mortality has decreased from 90% to 10–20%.20–25
Plasmapheresis is not, however, a harmless procedure. The high blood flow needed for plasma exchange make necessary the insertion of a central venous catheter, a high-risk procedure in patients with thrombocytopenia, with records of hemorrhage after insertion. Infectious complications are also a known complication of plasmapheresis. Other major complications recorded are pneumothorax, catheter thrombosis requiring removal of the central venous catheter, venous thrombosis requiring anticoagulant treatment, hypoxia and hypotension, and serum sickness.6
There is no standardization or commercialization of the tests that quantifies ADAMTS13 activity at most centers in Brazil. Due to high costs10,11,26 (around US$1400 each test) and delay in results (around 14 days), access to the pathophysiological basis to the diagnosis is difficult in developing countries, where high costs for plasma exchange, approximately US$300 each session (only equipment costs), and scarcity of specialized centers lead to the necessity for an accurate procedure indication.
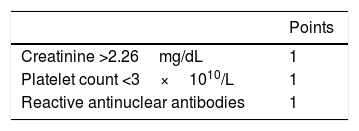
Diagnostic scores have been evaluated in the past few years, aiming to increase clinical diagnosis accuracy and guide early treatment for TTP patients.3,10,26 The PLASMIC score (Table 1) has been shown to be a useful tool as it has been able to diagnoses thrombotic microangiopathy associated with ADAMTS13 deficiency in a practical and statistically accurate manner. Other proposed scores are the French registry-based score27 (Table 2) and the one proposed by Bentley et al.28
The PLASMIC score – modified.26
| Points | |
|---|---|
| Platelet count <30×109/L | 1 |
| Hemolysis variables (reticulocyte count elevation; undetectable haptoglobin, or bilirubin – indirect >2.0mg/dL) | 1 |
| No active cancer | 1 |
| No history of solid-organ or stem-cell transplant | 1 |
| MCV <90fL | 1 |
| INR <1.5 | 1 |
| Creatinine <2.0mg/dL | 1 |
INR: international normalized ratio; MCV: mean corpuscular volume.
The French score – modified.27
| Points | |
|---|---|
| Creatinine >2.26mg/dL | 1 |
| Platelet count <3×1010/L | 1 |
| Reactive antinuclear antibodies | 1 |
The Plasmic score,26 was derived from the Harvard TMA Research Collaborative records, from Jan. 2004 to Dec. 2015 that included 214 patients with thrombotic angiopathy (TMA). It identified 7 variables (Table 1) able to clinically predict severe ADAMTS deficiency, with an AUROC from 0.91 (external validation) to 0.95 (internal validation).
The French score,27 was based on a cross-sectional analysis of the French TMA reference center records from 2000 to 2007. It identified three predictive variables (Table 2) for severe ADAMTS13 deficiency. In their study, the AUROC was 0.911.
MethodsStudy designA retrospective study was done in a public hospital in the Northeastern region of Brazil. Medical records of patients with the diagnosis of thrombotic microangiopathy (defined as the presence of thrombocytopenia and intravascular hemolytic anemia) and ADAMTS13 activity assay results from 2012 to 2016 were analyzed.
The ADAMTS13 activity and other tests were retrieved from medical records. Blood samples were collected at admission, before plasmapheresis, and sent for analysis at the Mayo Clinic Laboratories for the ADAMTS13 dosage by the ADMFX Fluorescence Resonance Energy Transfer (FRET). Other tests were performed at the hospital.
PatientsInclusion criteriaThe main inclusion criteria was the diagnosis of thrombotic microangiopathy (defined as the presence of thrombocytopenia and intravascular hemolytic anemia).
Exclusion criteriaWe excluded patients whose dosage of ADAMTS13 had not been performed and those whose medical records did not allow for the diagnosis of thrombotic microangiopathy.
MethodAt our hospital, most of the patients with a TMA hypothesis are referred from obstetric emergency or from other hospitals. Once the hypothesis of TMA is made, samples are collected for a complete blood count (CBC) with a peripheral blood smear, investigating schizocytes, blood clotting analysis, renal function, hepatic enzymes and antinuclear antibodies studies.
After the confirmation of the TMA, an additional sample is collected for ADAMTS13 activity assays and the state team responsible for the plasmapheresis is activated. In those cases in which plasmapheresis is not readily available, fresh frozen plasma is used until the apheresis is begun.
Data analysisPatients were divided into two groups using the original PLASMIC score criteria: ADAMTS activity less than 10% (patients with TTP) and greater than 10% (patients with a non-TTP TMA). The patients were then classified according to the PLASMIC score and then the correlation between the PLASMIC score and ADAMTS13 activity was evaluated to determine sensitivity, specificity and diagnostic accuracy, using the area under the ROC presence curve.
The correlation between the PLASMIC and the French score was also analyzed. We could not apply the score proposed by Bentley et al. because of missing test results.
Clinical and laboratorial features were analyzed according to the t-Test to independent samples and Fischer's exact test.
EthicsThe present study was structured in accordance with the Brazilian national ethical aspects and has been approved by the local ethics committee.
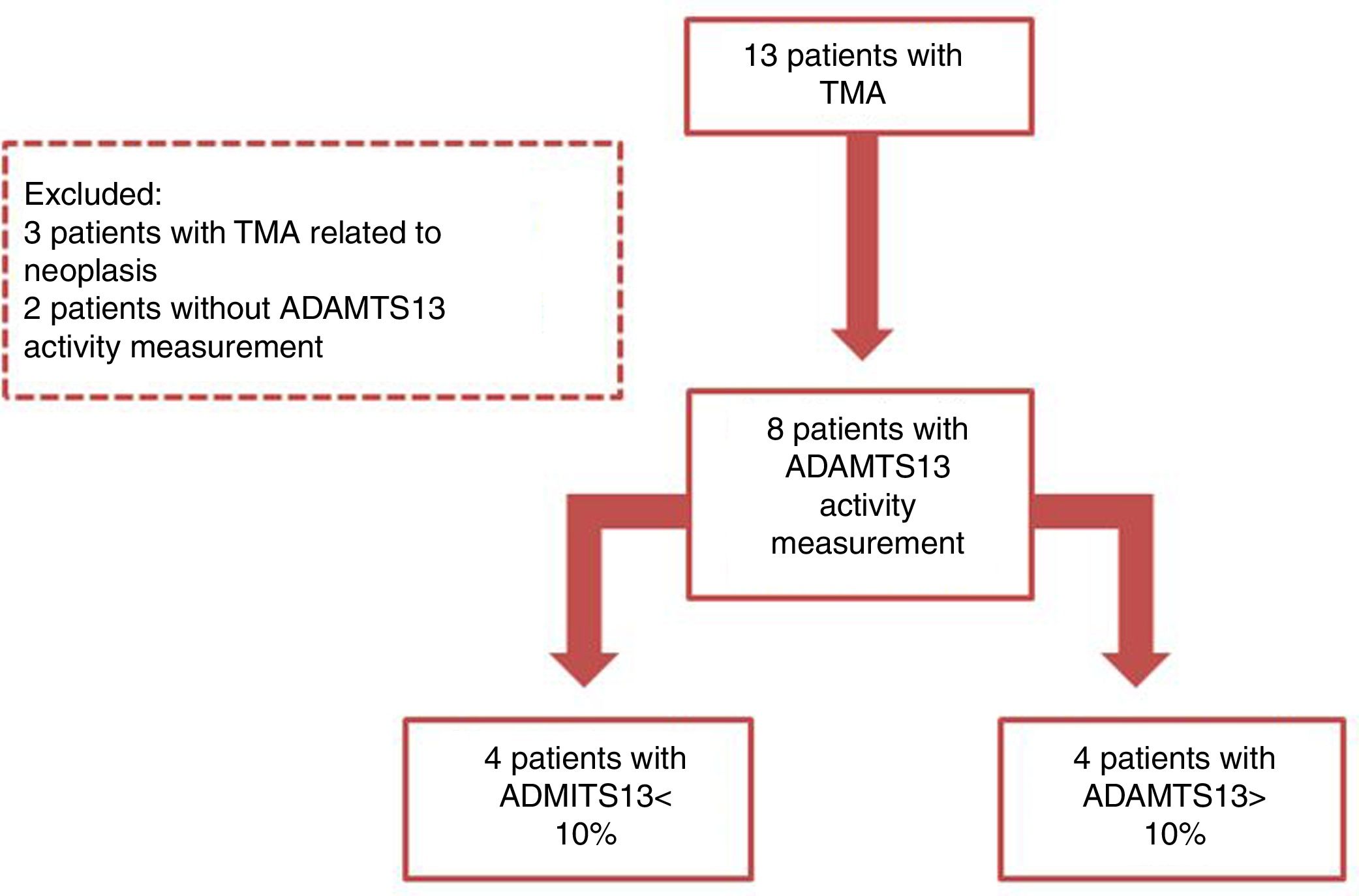
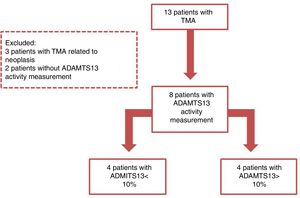
ResultsThirteen cases of thrombotic microangiopathy were registered during the study period. Three of these were cancer-related and have not been included in this study because the ADAMTS13 activity was not measured. Among the remaining cases, eight had the ADAMTS13 activity registered in medical records and were included (Figure 1). None of the patients included had a history of solid organ or hematopoietic stem-cell transplant in their medical records.
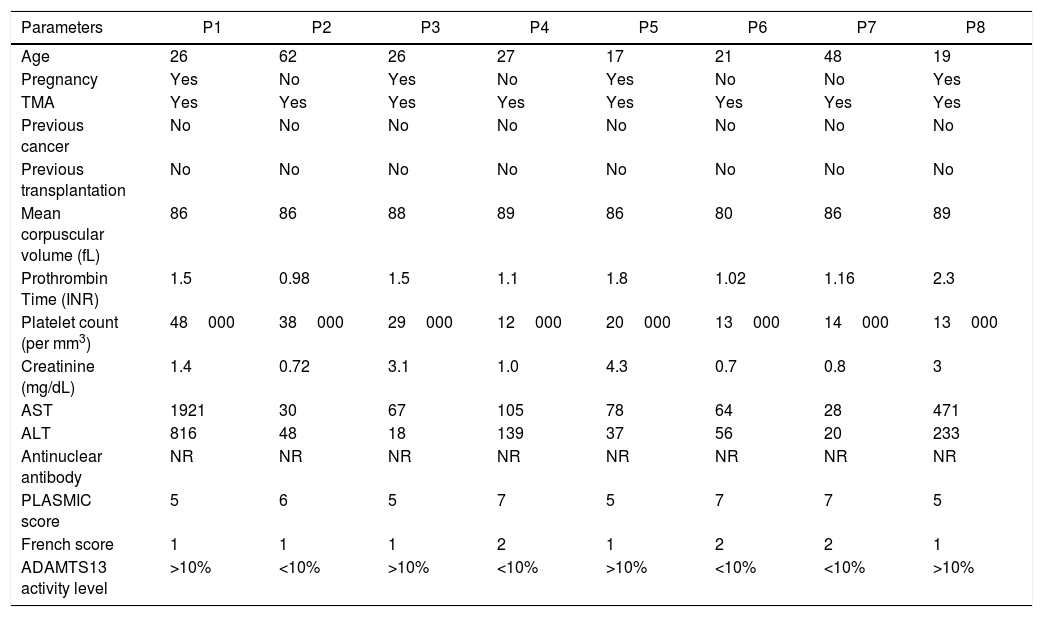
A summary of clinical and laboratorial characteristics, as well as patient treatment, can be seen in Table 3.
Collected data parameters.
| Parameters | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 |
|---|---|---|---|---|---|---|---|---|
| Age | 26 | 62 | 26 | 27 | 17 | 21 | 48 | 19 |
| Pregnancy | Yes | No | Yes | No | Yes | No | No | Yes |
| TMA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Previous cancer | No | No | No | No | No | No | No | No |
| Previous transplantation | No | No | No | No | No | No | No | No |
| Mean corpuscular volume (fL) | 86 | 86 | 88 | 89 | 86 | 80 | 86 | 89 |
| Prothrombin Time (INR) | 1.5 | 0.98 | 1.5 | 1.1 | 1.8 | 1.02 | 1.16 | 2.3 |
| Platelet count (per mm3) | 48000 | 38000 | 29000 | 12000 | 20000 | 13000 | 14000 | 13000 |
| Creatinine (mg/dL) | 1.4 | 0.72 | 3.1 | 1.0 | 4.3 | 0.7 | 0.8 | 3 |
| AST | 1921 | 30 | 67 | 105 | 78 | 64 | 28 | 471 |
| ALT | 816 | 48 | 18 | 139 | 37 | 56 | 20 | 233 |
| Antinuclear antibody | NR | NR | NR | NR | NR | NR | NR | NR |
| PLASMIC score | 5 | 6 | 5 | 7 | 5 | 7 | 7 | 5 |
| French score | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 |
| ADAMTS13 activity level | >10% | <10% | >10% | <10% | >10% | <10% | <10% | >10% |
INR: international normalized ratio; AST: aspartate aminotransferases; ALT: alanine aminotransferase; NR: non-reactive.
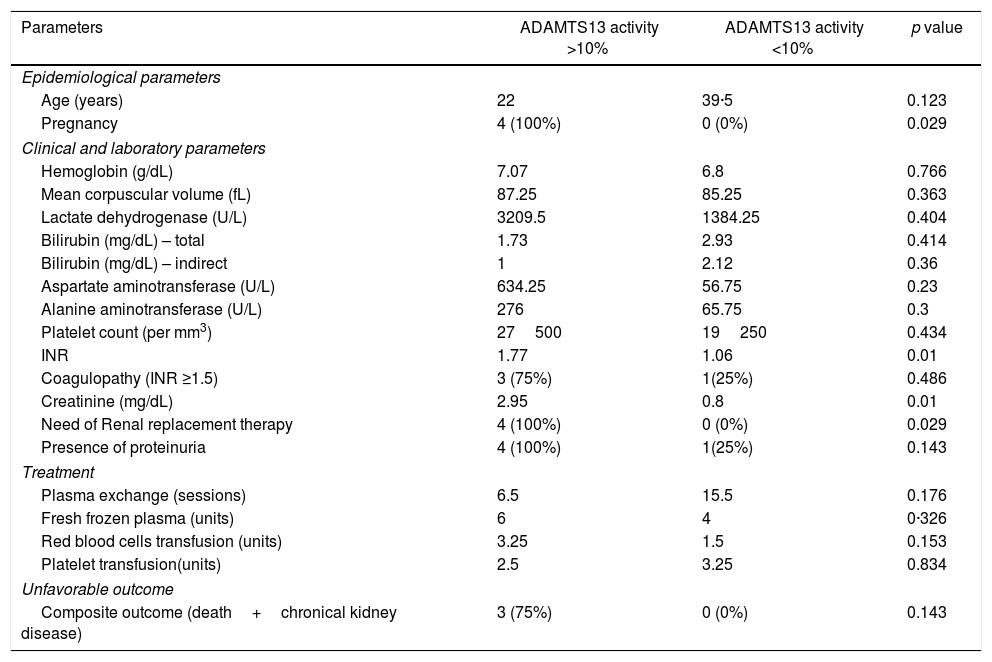
Patients in the group with ADAMTS13 activity under 10% had a mean age of 39.5 years; hemoglobin of 6.8g/dL; MCV of 82.5fL; LDH of 1384U/L; platelet count of 19250/mm3, and; INR of 1.06 (Table 4). In this group of patients, none had acute kidney injury at admission, however one of the patients (25%) had proteinuria. Among the four patients in this group, all had a PLASMIC score equal to or greater than six.
Characteristics of patients according to the presence or absence of ADAMTS13 deficiency.
| Parameters | ADAMTS13 activity >10% | ADAMTS13 activity <10% | p value |
|---|---|---|---|
| Epidemiological parameters | |||
| Age (years) | 22 | 39·5 | 0.123 |
| Pregnancy | 4 (100%) | 0 (0%) | 0.029 |
| Clinical and laboratory parameters | |||
| Hemoglobin (g/dL) | 7.07 | 6.8 | 0.766 |
| Mean corpuscular volume (fL) | 87.25 | 85.25 | 0.363 |
| Lactate dehydrogenase (U/L) | 3209.5 | 1384.25 | 0.404 |
| Bilirubin (mg/dL) – total | 1.73 | 2.93 | 0.414 |
| Bilirubin (mg/dL) – indirect | 1 | 2.12 | 0.36 |
| Aspartate aminotransferase (U/L) | 634.25 | 56.75 | 0.23 |
| Alanine aminotransferase (U/L) | 276 | 65.75 | 0.3 |
| Platelet count (per mm3) | 27500 | 19250 | 0.434 |
| INR | 1.77 | 1.06 | 0.01 |
| Coagulopathy (INR ≥1.5) | 3 (75%) | 1(25%) | 0.486 |
| Creatinine (mg/dL) | 2.95 | 0.8 | 0.01 |
| Need of Renal replacement therapy | 4 (100%) | 0 (0%) | 0.029 |
| Presence of proteinuria | 4 (100%) | 1(25%) | 0.143 |
| Treatment | |||
| Plasma exchange (sessions) | 6.5 | 15.5 | 0.176 |
| Fresh frozen plasma (units) | 6 | 4 | 0·326 |
| Red blood cells transfusion (units) | 3.25 | 1.5 | 0.153 |
| Platelet transfusion(units) | 2.5 | 3.25 | 0.834 |
| Unfavorable outcome | |||
| Composite outcome (death+chronical kidney disease) | 3 (75%) | 0 (0%) | 0.143 |
Plasma exchange (PE) was performed on all patients in this group (mean of 15.5 sessions) associated with high-dose methylprednisolone (1g/day) for three consecutive days, followed by a maintenance treatment with 1mg/kg/day of prednisone for at least six weeks. Two patients needed salvage therapy with anti-CD20 monoclonal antibody following the plasma exchange, one of them due to relapse after the initial steroid and PE cycle and the other, refractoriness. None of these patients have shown signals of a new microangiopathic episode or presented chronic kidney disease in the follow-up.
Among the patients with ADAMTS13 activity over 10%, the mean age was 22 years old; hemoglobin 7g/dL; MCV of 87.25; LDH 3209.5U/L; Platelet count of 27500/mm3 and INR of 1.77 (Table 4). All of these patients presented acute kidney injury and protein in the urinary sediment at admission. The four patients of this group were pregnant and two of them presented fetal death. All four patients in this group had the PLASMIC score under six and a final diagnosis of pregnancy-related TMA.
Plasma exchange was performed in three of them (mean of 6.5 sessions), steroid therapy was used in only one patient (1mg/kg/day of prednisone). An unfavorable outcome (death or chronic kidney disease) was seen in 3 (75%) of the patients without ADAMTS13 activity deficiency. The only patient that was not among those had the presumptive diagnosis of atypical hemolytic-uremic syndrome (aHUS) and used eculizumabe, a C5 blocker.2,29–33
Using a PLASMIC score equal to or greater than six as a diagnostic for TTP, as suggested by the original PLASMIC score study,26 there is perfect correspondence between the score and the activity of ADAMTS13 in the study sample. The PLASMIC score was also capable of accurately predicting response to plasma exchange and the long-term unfavorable outcome risk in the sample.
Applying the French TMA Reference Center-based score27 on our study patients, we noticed that no one scored 0 or 3 points. All 4 patients without ADAMTS13 deficiency scored 1 point in the French score. One of the 4 patients with ADAMTS13 deficiency scored 1 point in the French score and the others scored 2 points.
Using a cut-off of two points to propose ADAMTS13 deficiency, the area under the ROC curve was 0.9, with a sensitivity of 75% and specificity of 100%. The correspondence index between the PLASMIC and French scores was 87.5%.
DiscussionThe clinical diagnosis of TTP is made with the suspicion of thrombotic microangiopathy (TMA) associated with a neurological disorder.2,7,8,10–14 However, ADAMTS13 activity assays are unavailable in many developing countries, making the confirmation of the diagnosis difficult.26 This leads to numerous false positive diagnoses, exposing patients to the risk of plasma exchange and generating a great cost to the health system. On the other hand, because of the rarity of TTP, there was nearly a 7.5-day delay for the clinical diagnosis and the initiation of first-line treatment of patients enrolled in this study. Furthermore, the delay in diagnosis and treatment of TTP is associated with a worse outcome.3,5–7
In attempts to solve this problem, prognostic scores are now being used to reduce the chance of mistakes and increase the clinical diagnosis accuracy. Among the scores developed for the TTP diagnosis, the PLASMIC score has been shown to be practical and effective, according to validation studies.11,26
The application of the PLASMIC score showed practical and statistical superiority in our sample. An additional advantage of the PLASMIC score in comparison to the French and the Bentley et al. scores3,26,28 is the simplicity and availability of the exams it comprises, since the other scores need extra laboratory evaluation, which is not always available.
Moreover, the reproducibility of this instrument was quite satisfactory in our group of patients because it was able to accurately discriminate between patients who had ADAMTS13 deficiency and the group with normal enzyme activity (AUC: 1). The PLASMIC score can be very useful for the early diagnosis and initiation of an early adequate therapy, as well as indicating which patients will benefit more from treatment.
In summary, our study indicates that the PLASMIC score was effective in predicting reduced levels of ADAMTS13 activity, as well as discriminating patients without deficiency. It should be noted that in developing countries low-cost, fast, and effective instruments in predicting patients with TTP are important in the initial approach for TMA.
1LimitationsThis was a retrospective study and as such, no firm inference can be made concerning the impact of the use of this score in predicting treatment responsiveness and prognosis. More studies are needed to evaluate the reproducibility of this score in patients admitted with TMA in a prospective way and measure the real impact on prognosis.
ContributorsHFS was the chief investigator and DSO and TGL were principal investigators. HFS, DSO and TGL had the idea for the study and designed it. DSO, SATB, MAO and NPB collected data and did the data preparation and initial interpretation. TGL and FLNB did quality control and statistical analyses, checked the results and contributed to the data interpretation. HFS and SATB revised the data and critically revised the manuscript. All authors were involved in the interpretation of the data. All authors revised and approved the final report.
Conflicts of interestDSO has received grants from Alexion Pharmaceuticals – Brazil and MSD – Brazil.
HFS has received grants from Alexion Pharmaceuticals – Brazil.
TGL, FLB, SATB, MAO and NPB declare no competing interests.
Alexion Pharmaceuticals – Brazil funded the ADAMTS13 activity assays.
We would like to express our thanks to the Medicine Course of UNIFOR (University of Fortaleza), César Cals General Hospital, especially the Internal Medicine and Hematology Departments, and to HEMOCE (Hematology and Hemotherapy Center of Ceará), especially to all the professionals in the Plasma Exchange Department.