A new type of Coronavirus was identified by Chinese authorities in mid-December 2019, named by the Coronavirus Study Group of the International Commission on Virus Classification as “Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)” and the disease was named by the World Health Organization (WHO) as coronavirus disease-2019 (COVID-19). The new virus quickly spread around the world, being declared a pandemic in March 2020.1
The published data have focused on severe respiratory manifestations, found predominantly in adults, while in children the clinical manifestations are mostly asymptomatic and mild. When the disease is more severe in children, it occurs more frequently in those with less than one year of age or with pre-existing illnesses.1
Hematological changes are frequent in the COVID-19 disease, such as early lymphopenia and, as the disease progresses, anemia and neutrophilia.2 Thrombocytopenia can occur secondary to sepsis, disseminated intravascular coagulation or drug-induced,3 as well as direct bone marrow suppression or immune-mediated destruction.4
We report the case of a previously healthy child infected with SARS-CoV-2 who developed thrombocytopenia at two different times: during the acute infection and later, as immune thrombocytopenia (ITP), with complete response to intravenous immunoglobulin (IVIG).
Case reportA previously healthy two-year-old girl developed fever and bruises on her lower limbs, with no other clinical manifestations. Her platelets were 56,000/mm3, leukocytes, 3,100/mm3, neutrophils, 930/mm3, lymphocytes, 1,922/mm3 and C-reactive protein, 20.5 mg/L (Day 1 – D1). Her father had confirmed diagnosis of COVID-19. The child had a positive reverse transcription polymerase chain reaction (RT-PCR) for SARS-CoV-2, normal chest tomography, without the need for specific treatment. She recovered from the fever and on the third day of the follow-up, the platelet count rose to 108,000/mm3 (D3).
After 25 days of the diagnosis of COVID-19, she presented bruises and petechiae throughout her body, platelet count at 28,000/mm3 and no neutropenia. Serologies for HIV, hepatitis A and B, Epstein Barr virus and cytomegalovirus were non-reactive. The hepatitis B surface antibody (anti-HBs) and hepatitis A virus antibody (anti-HAV) were reactive secondary to vaccines. The antinuclear factor was non-reactive. Therefore, the diagnosis of newly-diagnosed ITP was made. After eight days, the bruises and the petechiae increased and platelets were at 16,000/mm3. She was admitted to the hospital to collect bone marrow aspirate and to receive IVIG at a programmed dose of 1 g/Kg/day for two days (D33).
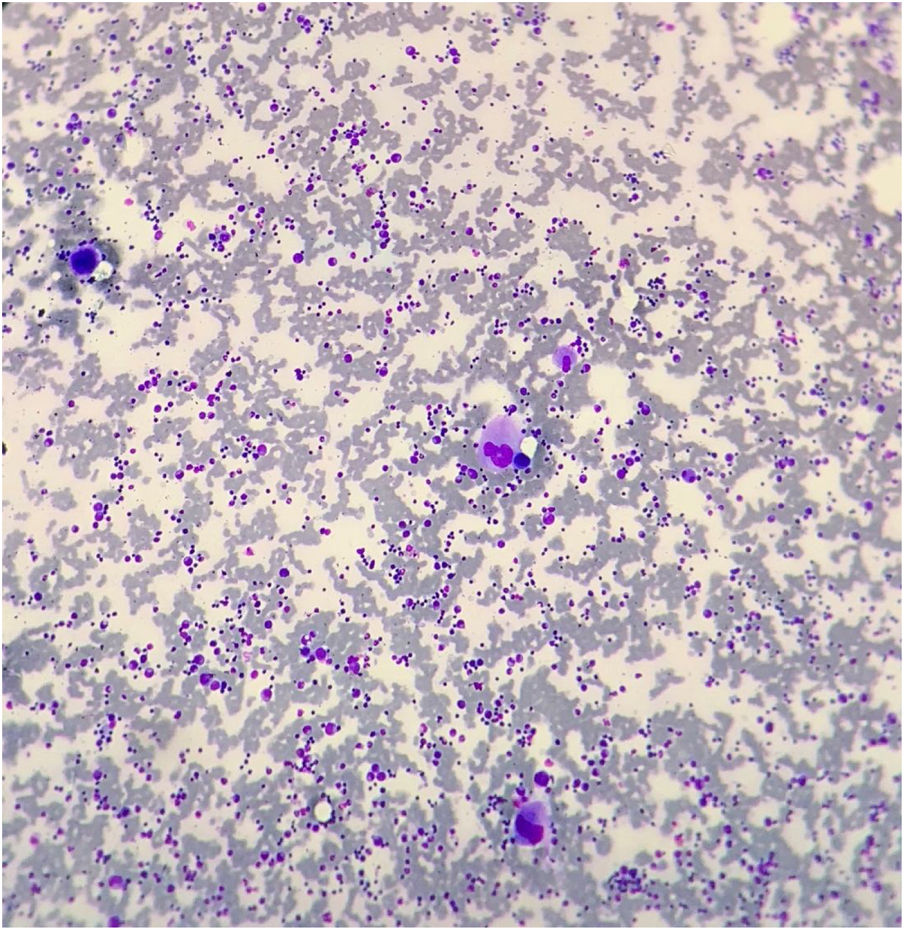
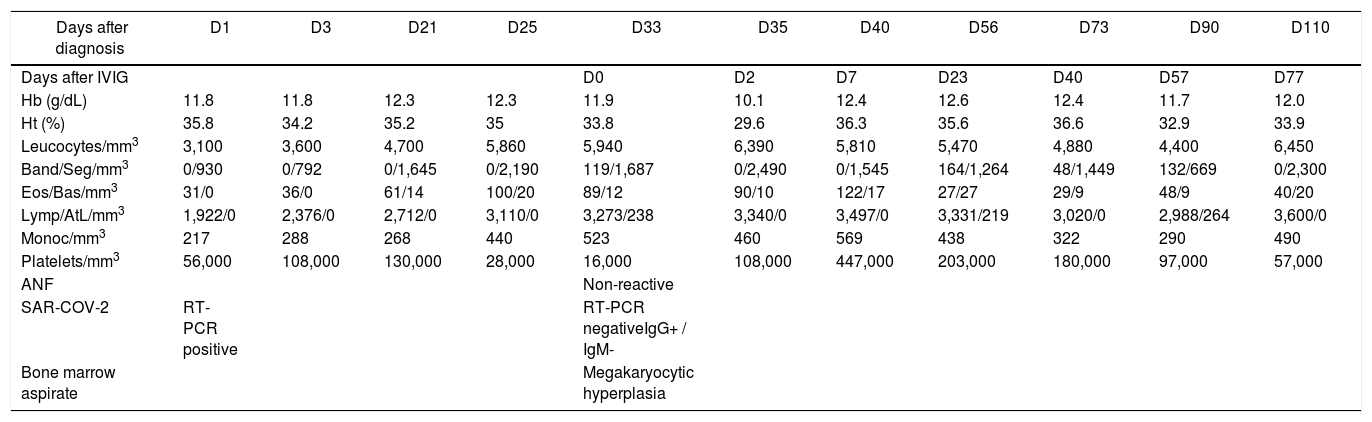
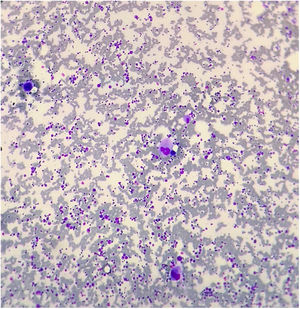
The bone marrow aspirate showed megakaryocytic hyperplasia and normocellularity of the erythrocytic and granulocytic series (Fig. 1). The RT-PCR for SARS-CoV-2 was negative and the serology for SARS-CoV-2 revealed negative IgM and positive IgG. After 48 hours of IVIG, the platelet count was 108,000/mm3 (D35). After five days (D40), the platelet count normalized (447,000/mm3), remaining normal until the D73 (D40 after IVIG) control, showing complete response to IVIG. On D87 she presented a runny nose and sneezing and on D90 (D57 after IVIG), the blood cell count revealed 97,000/mm3 platelets and atypical lymphocytes. No ITP treatment was administered, as there was no bleeding. The laboratory tests are described in Table 1.
Laboratory tests.
| Days after diagnosis | D1 | D3 | D21 | D25 | D33 | D35 | D40 | D56 | D73 | D90 | D110 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Days after IVIG | D0 | D2 | D7 | D23 | D40 | D57 | D77 | ||||
| Hb (g/dL) | 11.8 | 11.8 | 12.3 | 12.3 | 11.9 | 10.1 | 12.4 | 12.6 | 12.4 | 11.7 | 12.0 |
| Ht (%) | 35.8 | 34.2 | 35.2 | 35 | 33.8 | 29.6 | 36.3 | 35.6 | 36.6 | 32.9 | 33.9 |
| Leucocytes/mm3 | 3,100 | 3,600 | 4,700 | 5,860 | 5,940 | 6,390 | 5,810 | 5,470 | 4,880 | 4,400 | 6,450 |
| Band/Seg/mm3 | 0/930 | 0/792 | 0/1,645 | 0/2,190 | 119/1,687 | 0/2,490 | 0/1,545 | 164/1,264 | 48/1,449 | 132/669 | 0/2,300 |
| Eos/Bas/mm3 | 31/0 | 36/0 | 61/14 | 100/20 | 89/12 | 90/10 | 122/17 | 27/27 | 29/9 | 48/9 | 40/20 |
| Lymp/AtL/mm3 | 1,922/0 | 2,376/0 | 2,712/0 | 3,110/0 | 3,273/238 | 3,340/0 | 3,497/0 | 3,331/219 | 3,020/0 | 2,988/264 | 3,600/0 |
| Monoc/mm3 | 217 | 288 | 268 | 440 | 523 | 460 | 569 | 438 | 322 | 290 | 490 |
| Platelets/mm3 | 56,000 | 108,000 | 130,000 | 28,000 | 16,000 | 108,000 | 447,000 | 203,000 | 180,000 | 97,000 | 57,000 |
| ANF | Non-reactive | ||||||||||
| SAR-COV-2 | RT-PCR positive | RT-PCR negativeIgG+ / IgM- | |||||||||
| Bone marrow aspirate | Megakaryocytic hyperplasia |
ANF: Antinuclear factor; AtL: atypical lymphocytes; Band: band neutrophils; Bas: basophils; D: day; Eos: eosinophils; Hb: hemoglobin; Ht: hematocrit; IVIG: intravenous immunoglobulin; Lymp: lymphocytes; Monoc: monocytes; Seg: segmented neutrophils.
The Research Ethics Committee approved the case report and the Informed Consent Form was signed by the parents. Clinical and laboratory data were obtained from the patient's medical record.
Results and discussionSystematic review of 7,780 pediatric patients with COVID-19 did not find bruises, petechiae, thrombocytopenia or neutropenia as disease manifestations.5 Therefore, the patient presented a clinical condition different from the usual, reporting skin bleeding as the initial symptom, with thrombocytopenia and neutropenia appearing as laboratory test results. As already described in the literature for most symptomatic pediatric cases with COVID-19, the clinical and laboratory manifestations are mild,1,3 as observed in the reported patient. The purpose for the hospitalization was to treat ITP.
In adults, initial thrombocytopenia is more common, being related to a worse prognosis and occurring relatively frequently in cases requiring hospitalization for SARS-CoV-2. Thrombocytopenia in the late stage of the disease, 14 days after the onset of symptoms, is less frequent (11.8%) and lasts for approximately seven days, being in most cases transient.6
In the reported case, three weeks after the diagnosis of COVID-19, the patient developed clinical and laboratory characteristics of newly-diagnosed ITP. Other viral infections as the cause of ITP were excluded and the only factor associated was the previous viral infection by SARS-CoV-2. It is well established that newly diagnosed ITP in children is usually preceded by viral infections in the month prior to its onset.7
Considering that the natural evolution of ITP in children is remission in up to 12 months, the management of children with newly-diagnosed ITP consists of careful observation, regardless of the platelet count if there is only skin bleeding, or immunomodulatory treatment. First-line therapy for patients with newly-diagnosed ITP, platelets < 20,000/mm3 and active bleeding includes IVIG, corticosteroids or anti-D.7
In our case, as the child had a recent SARS-CoV-2 infection, whose hematological consequences are still poorly known in the pediatric population, we chose to perform the laboratory investigation and IVIG, with a complete response, maintaining a normal platelet count up to the D40 control after IVIG. The new thrombocytopenia after 2 months was not due to the loss of response to IVIG, but probably, to the new viral condition. In pediatric ITP, once the illness is resolved, signs of recurrence are possible, especially following another viral illness or antigen exposure, and the management of recurrent episodes is the same as that for newly-diagnosed cases.8
Until now, we found only one report of the occurrence of ITP after SARS-CoV-2 infection in a 10-year-old child, with a good response to treatment with IGIV. The ITP also occurred three weeks after infection by SARS-CoV-2, with petechiae on palate, lower limbs, chest and neck. The blood count had leukopenia, mild neutropenia and significant thrombocytopenia (5,000/mm3).9 More reports on cases of COVID-19 and ITP in adults have been published.3,10
Xu et al. proposed three mechanisms by which thrombocytopenia can occur in COVID-19: 1) reduction of platelet production by medullary invasion of the virus, inhibiting hematopoiesis, as well as by the reduction of hematopoietic precursors secondary to the cytokine storm; 2) increased platelet destruction due to autoantibodies and immune complexes, and; 3) increased consumption of platelets due mainly to lung injury, generating microthrombi in the circulation and thus increasing their consumption.4 The newly-diagnosed ITP typically occurs in healthy children, in whom platelet-mediated immune destruction can be triggered by a viral infection or other immunological phenomenon.11 Therefore, the characteristic immune mechanism of ITP may be secondary to infection by SARS-CoV-2.
During the SARS-CoV-2 pandemic, it is important to remember the association between isolated thrombocytopenia and COVID-19 and to include the RT-PCR for SARS-CoV-2 in the laboratorial investigation and follow-up, as ITP can develop. As much remains unknown about SARS-CoV-2, it is recommended to better understand the behavior of SARS-CoV-2 infection in pediatric patients.
Conflict of interestThe author declares no conflicts of interest.
We thank the patient and her parents for their willingness to share this case report.