Audit and education are essential pre-requisites in the review and update of blood transfusion practices. Although standard guidelines on appropriate utilization of blood components exists, erroneous use of blood components with no justification still continues. This study evaluates appropriateness of blood transfusion in obstetric settings and identifies key areas requiring educational intervention to improve blood transfusion practice toward the evidence-based at our hospital.
MethodThis was a prospective observational study on the analysis of blood transfusion requisition forms for obstetric patients before and after educational intervention, performed in two Phases from September 2011 to August 2012 and October 2012 to September 2013. The appropriateness of blood utilization was assessed against the Royal College of Obstetricians and Gynecologists Guidelines for blood transfusion and Green-Top Guideline no. 47. Data required for the study were obtained from department records and statistical analysis was performed using the SPSS, version 20 (IBM, USA).
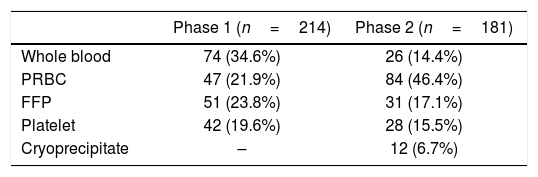
ResultsThe total transfusion episodes were 214 in 51 patients and 181 in 43 patients in Phases 1 and 2, respectively. Fresh frozen plasma was the most misused blood component, next to whole blood in Phase 1. However, appropriate utilization of components, including cryoprecipitate (6.6%), reduction in whole blood (34.5–14.4%) and single unit transfusion (23.3–18.2%) were observed in Phase 2. Inappropriate use of blood components, namely, packed red blood cells and fresh frozen plasma dropped significantly (p<0.05) from 29.7% to 13.1% and 45.1% to 22.5%, respectively, with the exclusion of platelet concentrate (33.3–20.6%, p-value 0.414).
ConclusionAudit and targeted education helped in optimizing transfusion practices in the obstetric setting.
Blood transfusion is often performed as a life-saving intervention in obstetrics. According to the sample registration system (SRS) report for 2014–2016, the maternal mortality rate in India accounts for 130 per 100,000 live births, of which 25–30% are due to obstetric hemorrhage and 15% is due to anemia.1–3 Hence, the World Health Organization (WHO) has recognized blood transfusion, known to reduce the maternal mortality rate when appropriately used,4 as one of the eight essential components of comprehensive emergency obstetric care (cEmOC).
It is not uncommon to observe a wide variation in blood transfusion practices among clinicians, as well as the indiscriminate and persistent use of whole blood and other blood components. This is most likely due to a lack of awareness in choosing the right component at the right dosage at the right time for the right patient. However, concerns about safety, cost, availability of blood components and associated risks and complications, such as transfusion reactions, transmission of infections and alloimmunization, have promoted the need for greater scrutiny of blood transfusion practice.5 Furthermore, a prompt decision in patient blood management, such as the selection of appropriate blood components or adoption of conservative strategies based on clinical and laboratory evaluation, including point-of-care coagulation tests, such as thromboelastography (TEG), and the continuous monitoring of transfusion activity through effective communication with the transfusion team, may help to improve transfusion practice. Therefore, we planned to evaluate the appropriateness and rational use of blood components in the obstetrics setting at our center and, in the light of the results, to identify key areas which require educational intervention to improve blood transfusion practices, based on the clinical evidence obtained at our hospital.
MethodsA prospective cross-sectional study on the analysis of blood transfusion requisition forms and transfusion details of obstetric patients for a period of 18 months in two phases (Phase I – September 2012 to March 2013 and Phase II – August 2013 to March 2014) was conducted in the Department of Transfusion Medicine at our hospital. Approximately twenty-five clinicians from the Department of Obstetrics and Gynecology, of designations ranging from professor to junior resident, consented to include them in this study. The Institutional Ethical Committee clearance (IEC/11/92) was sought before the commencement of the study and an informed consent waiver was approved by the IEC to evaluate transfusion request forms of obstetric patients during the study period. The transfusion requests received for whole blood (WB) and other blood components, such as packed red blood cells (PRBCs), fresh frozen plasma (FFP), platelet concentrate and cryoprecipitate, for obstetric patients during the study period were reviewed for appropriateness and rational use against the Royal College Of Obstetricians and Gynecologists (RCOG) guidelines for blood transfusion in obstetrics, Green-Top Guideline no. 47.6 Despite the availability of alternative treatment modalities, utilization of blood components without any indication or justification, if noted, was also considered inappropriate in this study. Transfusion requests received from outside hospitals were excluded. The variables, such as patient demographic details, blood group, parity, gestational age, hemoglobin level, platelet count, coagulation profile, provisional diagnosis, indications for transfusion and type and number of blood components requested, were documented for every transfusion event from the transfusion request forms during both phases of the study. The principal investigator communicated the observations made in Phase 1 to the obstetric clinicians in their departmental meeting, with the aid of a power-point presentation. The key areas that require improvement were highlighted. Furthermore, continuous educational awareness emphasizing the properties of blood, the rationale to avoid whole blood transfusion, advantages of blood component therapy, benefits and risks of transfusion therapy, blood conservation strategies and evidence-based transfusion support, was provided to aid the clinicians in making correct blood prescriptions. This education happened at regular intervals for four months through scientific lectures, one-on-one discussions to clarify clinician doubts, prospective review and feedback on transfusion requisition forms. Subsequently, Phase 2 was monitored for the outcome of educational intervention as for change in blood transfusion practices toward appropriate selection and utilization of blood components. The data required for the study were obtained from department records and laboratory software. Collected data of both the Phases were compiled in Microsoft Excel for data analysis. Statistical significance of difference in blood transfusion between the two phases, i.e., a p-value <0.05 was analyzed by the Chi-square test χ2.
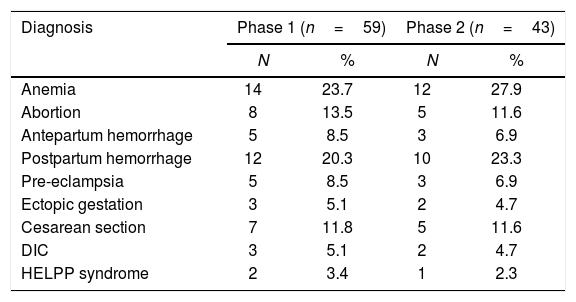
ResultsOf the total of 1298 obstetric admissions, 59 patients in Phase 1 and 43 patients in Phase 2 received blood transfusion accounting for the mean transfusion rate of 7.8%. The mean age of patients in Phases 1 and 2 was 24.6 and 22.4 years, respectively. The majority (61.2%) of transfused patients were multiparous while 38.7% were nulliparous women. The most common provisional diagnosis in both the Phases included anemia and postpartum hemorrhage, followed by others (Table 1). The total transfusion episodes were 214 and 181 in Phases 1 and 2, respectively. The order of blood utilization noted was whole blood (34.5%)>PRBCs (21.9%)>FFP (23.8%)>platelets (19.6%) in Phase 1 and PRBCs (46.4%)>FFP (17.1%)>platelets (15.4%)>whole blood (14.3%)>cryoprecipitate (6.6%) in Phase 2 after educational intervention (Table 2). At the end of Phase 1, the key areas identified for improvement include the provision of complete patient details, as requested on the blood ordering form, implementation of appropriate component therapy, rather than whole blood, in compliance with standard guidelines, and the adoption of a multidisciplinary approach in patient blood management.
Distribution of provisional diagnosis of patients in Phases 1 and 2.
| Diagnosis | Phase 1 (n=59) | Phase 2 (n=43) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Anemia | 14 | 23.7 | 12 | 27.9 |
| Abortion | 8 | 13.5 | 5 | 11.6 |
| Antepartum hemorrhage | 5 | 8.5 | 3 | 6.9 |
| Postpartum hemorrhage | 12 | 20.3 | 10 | 23.3 |
| Pre-eclampsia | 5 | 8.5 | 3 | 6.9 |
| Ectopic gestation | 3 | 5.1 | 2 | 4.7 |
| Cesarean section | 7 | 11.8 | 5 | 11.6 |
| DIC | 3 | 5.1 | 2 | 4.7 |
| HELPP syndrome | 2 | 3.4 | 1 | 2.3 |
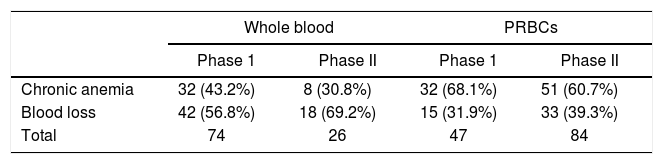
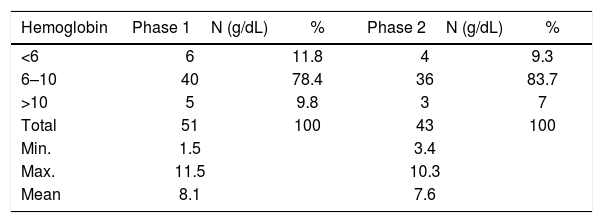
Although currently not recommended, whole blood utilization of 56.7% (42/74) in Phase 1 and 69.2% (18/26) in Phase 2 was observed in this study, the majority of which was used in the management of acute blood loss due to antepartum, intrapartum and post-partum hemorrhage and surgical blood loss. On the other hand, a significant proportion of PRBC transfusion in both Phases [68% (32/47) in Phase 1 and 60.7% (51/84) in Phase 2] was observed in anemic patients and included the pre-operative correction of anemia before cesarean section (Table 3). The proportion of appropriate PRBC transfusion in Phases 1 and 2 were 70.2% and 86.9%, respectively. The mean hemoglobin (Hb) trigger in Phases 1 and 2 were 8.3g/dL and 7.6g/dL, respectively, ranging from 1.5g/dL to 11.5g/dL. The majority of patients in both Phases had pre-transfusion Hb of 6 to 10g/dL (Table 4) and most of the inappropriate PRBC transfusion observed in this group was performed to achieve the target hemoglobin level in hemodynamically stable patients. Single unit transfusion of 43.2% (32/74) whole blood and 38.2% (18/47) PRBCs observed in Phase1 decreased to 34.7% (8/23) whole blood and 29.7% (25/84) PRBCs in Phase 2.
Among the FFP transfusions, 56.8% (22/51) and 80.6% (25/31) in Phases 1 and 2, respectively, account for appropriate transfusion, the common indication being the multiple clotting factor deficiency due to obstetric hemorrhage. However, FFP was identified to be the most misused blood product in this study, next to whole blood. The FFP transfusions of approximately 31.3% (16/51) in Phase 1 and 12.9% (4/31) in Phase 2 account for avoidable transfusion, as they were utilized for prophylactic correction of prolonged prothrombin time (PT) and/or activated partial thromboplastin time (aPTT), in the absence of active bleeding. Furthermore, 11.7% (6/51) in Phase 1 and 6.4% (2/31) in Phase 2, used in the management of hypovolemia, were also considered inappropriate. Utilization of cryoprecipitate, a product rich in fibrinogen concentrate, was observed only in Phase 2 to resuscitate patients with massive blood loss (Table 2).
Platelet concentratePlatelet transfusions of 71.4% (30/42) in Phase 1 and 82.7% (24/29) in Phase 2 were considered appropriate, as they had both therapeutic and prophylactic indications, such as thrombocytopenia due to obstetric hemorrhage, followed by pre-eclampsia, dengue and the hemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome. The mean pre-transfusion platelet count for prophylactic transfusion in Phase 1 was 52×109/L, whereas in Phase 2, it was 20×109/L. Platelet transfusions of 28.5% (12/42) in Phase 1 and 17.2% (5/29) in Phase 2 were considered inappropriate for thrombocytopenic patients with no risk factor for bleeding, including immune thrombocytopenia.
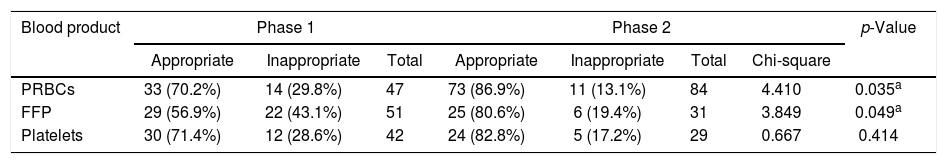
Overall, the proportion of inappropriate transfusions of PRBCs, FFP and platelets have decreased from 29.7%, 45.1% and 28.5% to 13.1%, 22.5% and 17.2%, respectively, in Phase 2 after the regular periodic educational intervention and were found to be statistically significant for all blood components, with a p-value <0.05, except for platelet concentrate (Table 5).
Appropriate and inappropriate blood utilization in Phases 1 and 2.
| Blood product | Phase 1 | Phase 2 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Appropriate | Inappropriate | Total | Appropriate | Inappropriate | Total | Chi-square | ||
| PRBCs | 33 (70.2%) | 14 (29.8%) | 47 | 73 (86.9%) | 11 (13.1%) | 84 | 4.410 | 0.035a |
| FFP | 29 (56.9%) | 22 (43.1%) | 51 | 25 (80.6%) | 6 (19.4%) | 31 | 3.849 | 0.049a |
| Platelets | 30 (71.4%) | 12 (28.6%) | 42 | 24 (82.8%) | 5 (17.2%) | 29 | 0.667 | 0.414 |
Optimal utilization of blood transfusion strategies and rational use of blood components are essential pre-requisites to ensure patient safety. The difference in transfusion practices among clinicians often reflects an expert opinion, tradition, community practice, personal experience or lack of awareness regarding the indication and risk associated with transfusion therapy.7–9 Therefore, it is essential to monitor transfusion practices and make them more clinical evidence-based.
The overall blood transfusion rate in this study accounts for 7.8%, which correlates with other similar studies conducted in India.5 Although the goal of modern transfusion practice is to provide appropriate replacement therapy with required components,10 34.6% of whole blood transfusion was observed in Phase 1, of which 43.2% is accounted for in the management of chronic anemia (Table 3). It is important to be aware that whole blood transfusion carries the risk of volume overload in chronic anemia patients and is indicated only in the management of acute blood loss, if components are unavailable. However, in Phase 2, after the educational intervention, whole blood usage has been significantly reduced to 14.3%. The FFP was observed to be the most misused blood component, next to whole blood. Nonetheless, auditing in Phase 2, followed by educational intervention, demonstrated a statistically significant reduction in inappropriate usage of all blood components, except platelet concentrate.
According to Green-Top Guideline no. 47, Royal College of Obstetricians and Gynecologists, on blood product replacement, packed red cell transfusion is rarely indicated with a hemoglobin level greater than 10gm/dL and is almost always indicated with a hemoglobin level less than 6g/dL. In patients with an intermediate hemoglobin level of 6–10g/dL, the rate and magnitude of blood loss, the patient's cardiopulmonary reserve and factors affecting oxygen consumption should be assessed before considering blood transfusion.6 The categorization of the patient's pre-transfusion hemoglobin level in our study showed that the majority of patients had an intermediate hemoglobin level between 6 and 10g/dL (Table 4), in light of which the factors mentioned above and transfusion medicine expert opinion play a crucial role in assisting the clinicians in making appropriate decisions on blood transfusion.
The PRBC transfusion in both the phases was performed mainly for the immediate correction of anemia. However, the correction of hypovolemia, owing to blood loss, and the attainment of the target hemoglobin were deemed inappropriate for 29.8% in Phase 1 and 13.1% in Phase 2. The mean Hb trigger of 8.4g/dL in Phase 1 dropped to 7.2g/dL in Phase 2 after avoiding unnecessary transfusion to hemodynamically stable, early and mid-trimester anemic patients. In a study by Parker et al., a low hemoglobin level, in the absence of symptoms, was reported as the most common reason for red cell transfusion, while other studies in the literature have documented different reasons for the inappropriate use of packed red blood cells.5,11,12
Single unit transfusion of both whole blood and PRBCs accounted for 41.3% (50/121) in Phase 1 and 30.8% (33/107) in Phase 2. Among these transfusions, 26.44% (32/121) in Phase 1 and 19.62% (21/107) in Phase 2 were actually identified as eligible for alternative conservative management, rather than single unit transfusion. In a study by Gupte et al., the incidence of single unit transfusion was observed at 30–40%.5 Early recognition of iron deficiency in the antenatal period, followed by iron therapy, could promptly reduce the single unit PRBC transfusion requirement in antenatal mothers.3
The FFP transfusion is generally indicated in single or multiple coagulation factors deficiency or correction of an elevated PT, aPTT associated with bleeding or acute disseminated intravascular coagulation (DIC) and in massive transfusion.13 In our study, 56.86% and 80.64% of the FFP transfusion in Phases 1 and 2 accounts for appropriate transfusion, but the FFP was found to be the most misused product in our study, as 43.13% in Phase 1 and 19.35% in Phase 2 were inappropriately used as a volume expander or an elevated PT and aPTT level corrector in non-hemorrhagic patients. Shingare et al. observed a similar finding, while a study by Barnette et al. mentioned that an educational awareness may help to reduce the inappropriate use of FFP,13,14 the same being reflected in our study.
The majority of platelet transfusions in both Phases were found to be appropriate. However, few transfusions (28.5%) in Phase 1, performed in thrombocytopenic patients with the diagnosis of platelet function defect or immune thrombocytopenia in the absence of active bleeding or risk factors for bleeding, account for inappropriate transfusion. This proportion decreased in Phase 2 after enlightening the clinicians about indications for platelet transfusion,6 yet was not statistically significant.
Of concern, patients with massive transfusion requirements in Phase 1 were managed with whole blood, packed red blood cells, fresh frozen plasma and platelets in no predefined ratio. Recent literature states that massive transfusion performed blindly on a fixed ratio basis may overtreat or undertreat the patient. Furthermore, it emphasizes that the activation of a massive transfusion protocol has to be evidence-based and in conjunction with either conventional or point-of-care coagulation testing, such as thromboelastography, to ensure appropriate utilization of blood products.15 Moreover, it is important to consider cryoprecipitate for those patients requiring massive transfusion with fibrinogen deficiency, as fibrinogen is an early predictor of the severity of post-partum hemorrhage.16 In our study, only after updating the clinicians with the above-mentioned findings, 6.68% of cryoprecipitate transfusion in the management of massive blood loss was observed in Phase 2.
The transfusion practice before educational intervention revealed the unfamiliarity of clinicians with the current clinical practice guidelines for blood and blood product transfusion. However, Phase 2 demonstrated a tremendous improvement in optimal utilization of blood components. Several transfusion audits in the literature have proved that blood transfusion practice can be improved by combining evidence-based transfusion triggers with the education of physicians.17,18 Furthermore, for sustained improvement, there must be a system in place to evaluate and monitor the blood transfusion practice on a routine basis.19,20 However, this study has several limitations, such as limited sample size and study setting, failure to include the confounding variables that may influence the transfusion requirement, including underlying systemic infection, hemonitics therapy or medication history, lack of external validation of educational intervention and failure to collect post-transfusion laboratory values to comment on transfusion efficiency. Nonetheless, this study was considered as a stepping stone to bring the desired change in transfusion practices toward the evidence-based at our center. Although the decision-making on the transfusion of blood or components is done by the treating physician, it would be very desirable if this resulted subsequent to the consultation with the transfusion specialist to ensure maximum benefit to the patient with minimal risk.
ConclusionThis study highlights that blood transfusion audits, when combined with education in the clinical setting, aid clinicians in optimizing the blood ordering and transfusion practices toward the rational and evidence-based. Furthermore, early intervention with a multidisciplinary approach in blood management in obstetric patients may help to reduce the maternal mortality rate.
Authors’ contributionsDhivya Kandasamy and Aswin Kumar contributed in conception and study design, data acquisition, analysis, interpretation and drafting of the manuscript. Joshua Daniel Jeyakumar helped in a critical review of the draft.
Conflicts of interestThe authors declare no conflicts of interest.