Antiphospholipid syndrome (APS) is an autoimmune disease characterized by the persistent presence of antiphospholipid (aPL) antibodies.1 Clinical consequences of APS include inflammatory venous or arterial thrombosis and/or pregnancy loss. APS can be classified according to its clinical manifestation as thrombotic and/or obstetric or, more rarely, catastrophic (CAPS), where there is life-threatening multiorgan involvement. The diagnosis of CAPS is established by Sapporo criteria plus preliminary classification criteria,1,2 consisting of identification of more than three documented thrombotic events (arterial, venous and small vessel thrombosis) in various organs over a less than one week interval, with autoimmunity confirmed by the presence of three different aPLs. The mortality rate of CAPS has been reported to approach 50% despite the initial of early treatment.3
Individuals infected with SARS-CoV-2, the virus responsible for COVID-19, have a heterogeneous and unpredictable course, from asymptomatic forms, oligosymptomatic with flu-like symptoms, to severe forms with atypical pneumonia and very severe forms with high mortality that may eventually develop APS antibodies, whose role in thrombosis associated with COVID-19 is controversial.4 We present a case of CAPS associated with oligosymptomatic SARS-Cov-2 infection in a patient attended in the emergency service of our hospital.5,6
Case presentationA 43-year-old man with a history of chronic alcoholism, hypertension, an autoimmune disease that had not been well documented and had been associated with seizures for the past two years but without treatment, and was admitted to the emergency room of our hospital in Quito, Ecuador, with a 48 hour medical history characterized by cognitive impairment that made daily activities difficult, decreased visual acuity diagnosed as acute encephalopathy. On admission, his vital signs were in the normal range. On physical examination, he was diagnosed with macroglossia and ulcerative lesions on the palate and oropharynx. He also possessed bilateral pitting edema in his lower limbs, ulcers on both legs, livedo reticularis, fluctuating changes in his state of consciousness, a cognitive impairment that hindered his daily activities and decreased visual acuity.
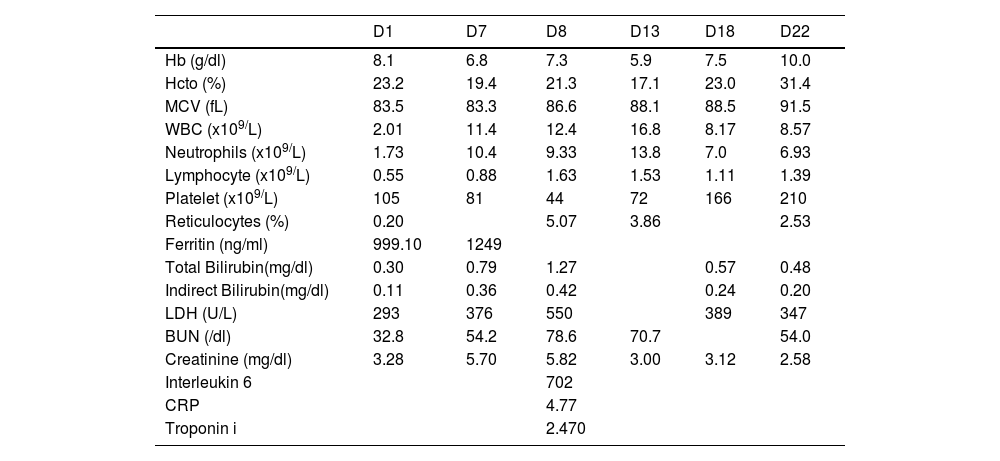
Laboratory studies revealed a hemoglobin of 8.1 g/dL (11.7–16.3 g/dL), a white blood cell count of 2.01 × 103/uL (3.56–10.30 × 103/uL), absolute neutrophils at 1.73 × 103/uL (2.00–6.40 × 103/uL), a lymphocyte level of 0.55 × 103/uL (1.00–3.20 × 103/uL), platelets count of 105 × 103/uL (167–431 × 103/uL), normal bilirubin levels and reticulocytes count of 0.20% (0.5–1.50%). A direct antiglobulin test (DAT) was positive, BUN was 32.8 mg/dL (18.0–45 mg/dl), his creatinine level was 3.28 mg/dL (0.5–0.9 mg/dl), lactate dehydrogenase was 293 U/L (135–214 U/L), ferritin 999.10 μcg/dL (20–150 μcg/dL), B12 Vitamin 2000 pg/mL (197.0–771.0 pg/mL) and folic acid 11.74 ng/mL (0.60–20.00 ng/mL). The diagnosis of alcoholic encephalopathy was tentatively proposed at admission. The patient mentioned being in close contact 72 h previously with an individual diagnosed with COVID-19. A nasopharyngeal swab samples was, therefore, obtained and was positive for SARS-CoV-2 by RT-PCR. The RT-PCR was repeated 72 h after the initial study, to rule out a false positive result, and confirmed the presence of viral RNA, despite the absence of any respiratory symptoms.
On day 4 of hospitalization, a brain tomography scan was performed and revealed a hypodense right parieto-occipital image compatible with chronic microvascular ischemia. Subsequent electroencephalogram and cerebrospinal fluid evaluations were normal. Studies to diagnose systemic lupus erythematosus (SLE) were negative, with ANA results by indirect immunofluorescence (IFI) titers less than 1/80, Anti DNA titers less than 1/10, C3 133.8 mg/dl and C4 14.2 mg/dl (not consumed), but the patient was positive for three antibodies associated with APS: the lupus anticoagulant (75.8 s), anticardiolipin IgG (>120 U/ml) and anti-β−2-glycoprotein IgG (>100 U/ml).
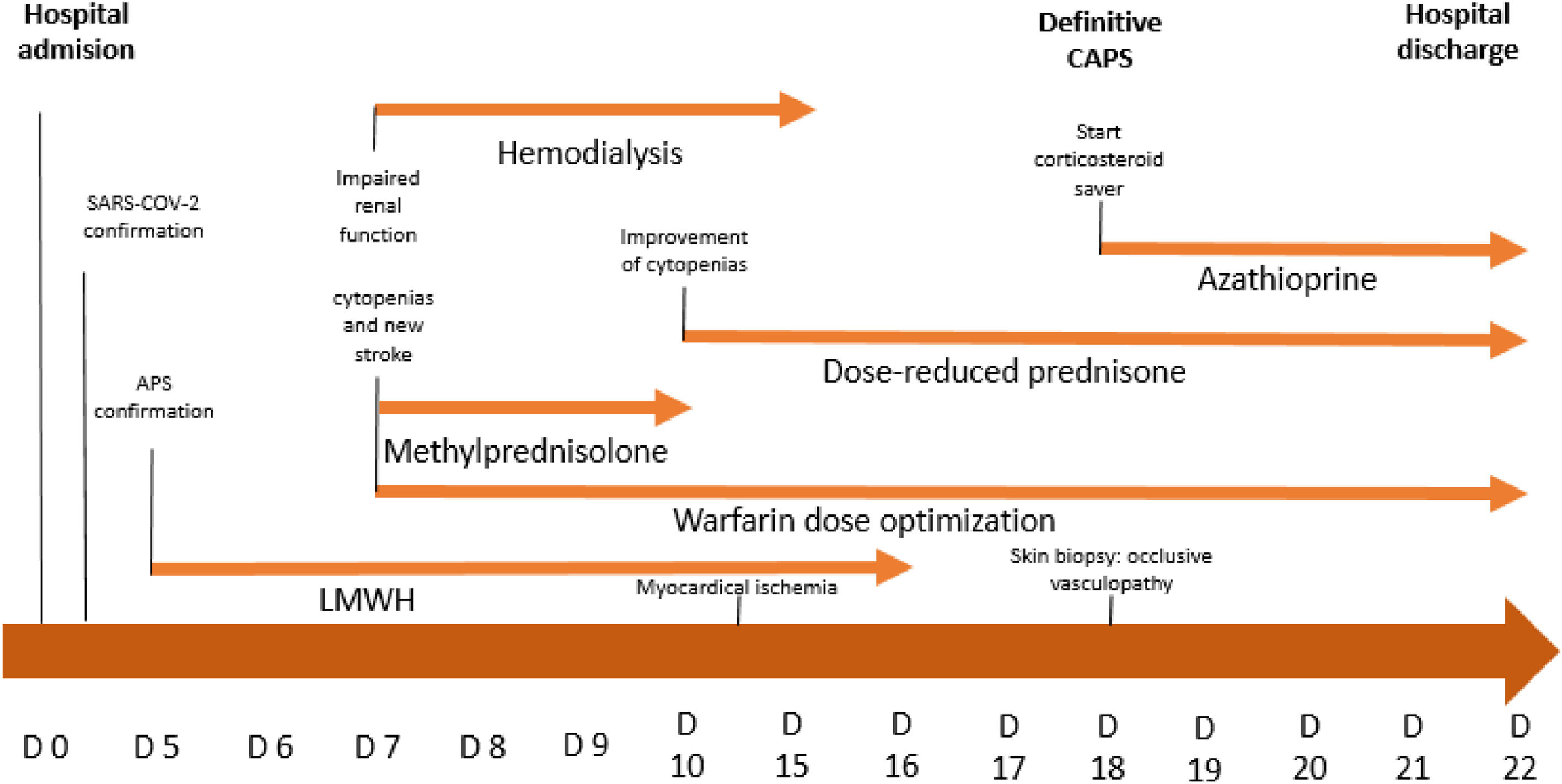
On day 5, treatment was initiated with low molecular weight heparin (LMWH) at 1 mg/kg every 12 h. Two days later the patient showed evidence of progressive deterioration in his level of consciousness, accompanied by a productive cough and dyspnea that progressed to respiratory failure, a worsening of renal function and the need for hemodialysis. Subsequently, mechanical ventilation was initiated, in association with red blood cell transfusion, meropenem and vancomycin coverage to treat septicemia. His ventilation parameters worsened, and there was a marked diminution of his state of consciousness that persisted even after suspending sedation. A MRI was performed and revealed areas of recent cerebral infarction alternated with areas of previous ischemia (Figure 1A,B,C,D).
Brain MRI. Diffusion images showing hyperintense areas consistent with multiple areas of cerebral ischemia (orange arrows). A. occipital lobe. B and C. temporal lobe. periventricular level. and D. parietal cortex. E and F: Skin biopsy electron microscopy: Vascular proliferation with segmental hyalinization of the wall. PAS-positive X40.
On admission to the ICU, laboratory tests revealed persistent cytopenias and the presence of markers of hemolysis. His hemoglobin was 6.8 mg/dl, platelets count was 81 × 103/L, reticulocytes were 5.07%, lactate dehydrogenase was 376 U/L, positive DAT persisted and inflammatory markers, C reactive protein 4.77 (0.00–0.79 mg/dl) and interleukin-6 702 (<7 pg/ml), were detected.
On day 7, a one-gram intravenous (IV) methylprednisolone pulse was started for three days, plus LMWH 1 mg/kg every 12 h plus warfarin 5 mg daily. This resulted in a clear improvement in hematological parameters (Table 1). His creatinine level also decreased. He was maintained without renal replacement therapy and developed a reduced need for intensive support. Subsequently, he was maintained on prednisone 1 mg/kg/day PO and a new immunosuppression therapy was initiated with azathioprine 150 mg/day, a corticosteroid-sparing agent, plus warfarin to obtain an International Normalized Ratio (INR) between 2 and 3.
Laboratory results of the patient during hospitalization.
BUN: Blood Urea Nitrogen; CRP: C-reactive protein; Hb: Hemoglobin; Hcto: Hematocrit; LDH: Lactate dehydrogenase; MCV: Medium Corpuscular Volume; WBC: White blood cell.
On day 13, due to marked hemodynamic instability plus signs of heart failure, an investigation of myocardial ischemia markers was performed. His troponin T was 2.470 pg/mL (<0.014 pg/ml) and an echocardiogram showed signs of left ventricular hypokinesia.
Since his hospitalization in the ICU it was possible to confirm his ischemic stroke, worsening renal function, and documented myocardial ischemia associated with positivity for three aPL antibodies. The patient met criteria for probable CAPS, and on day 18 of hospitalization (three thrombotic events at different sites: confirmed in CNS, cardiac, cutaneous, and unconfirmed renal, and retinal events, all present at an interval of less than one week, and presence of positive antiphospholipid antibodies). The definitive diagnosis of CAPS was confirmed with the pathology study of the ulcerative skin lesion, which showed a reaction of occlusive vasculopathy correlated with CAPS (Figure 1E,F).2
The patient did not have a recurrence of thrombotic events and achieved clinical stability, despite cognitive neurological sequelae and bilateral blindness that was highly suspected as a thrombotic event in the retinal artery, but could not be confirmed. He was discharged on day 22 (summary of event in Figure 2).
DiscussionThe present case met the definition of CAPS, but the existence of additional pathologies made the diagnosis difficult. This resulted in disease progression and negative sequela. During the patient's hospitalization, there was adverse systemic involvement in the central nervous, hematological, cardiovascular, and renal systems. CAPS is diagnosed predominantly in young females and in approximately 6 of 10 cases appears without the identification of any underlying autoimmune disease. When CAPS arises as a secondary manifestation, systemic lupus erythematosus, rheumatoid arthritis, or dermatomyositis are typically the primary pathologies. The present case, primary CAPS in a male patient, is a rare occurrence, the presence of recurrent thrombotic events and vasculopathy changes in a skin ulcer biopsy were compatible with vascular involvement due to CAPS, completing the Sapporo criteria.7 Thrombocytopenia is a relatively frequent finding in APS, but it is not considered part of the criteria for APS and CAPS, being found in only 20% of cases.8 The cause of thrombocytopenia is not fully elucidated, but may likely be a consequence of platelet consumption, which could explain the presentation in this case.9
An association between SARS-Cov-2 infection and the eventual presence of antiphospholipid antibodies has already been described, but the role of this association in the development of thrombotic events is still controversial.10,11 Our patient was diagnosed with CAPS at the peak of the COVID-19 pandemic and we were not able to find a direct relationship between oligosymptomatic infection and the presence of CAPS manifestations, as separating these diseases, the emerging and the old, is not an easy task. Additionally, many of the clinical and laboratorial features of our patient could have been erroneously attributed to SARS-Cov-2, as this infection is, in some cases, characterized as a hypercoagulable state.7 Although COVID-19 has been shown to induce the prothrombotic phenotype with endothelitis, the finding of aPL in these patients is novel and, its active participation in the pathogenesis of thrombosis is still under debate.12 Given that the mortality rate in CAPS is approximately 50% despite anticoagulant and immunosuppressive therapy, a high index of collaboration of a multidisciplinary medical team are vital for the survival of these patients.7 As CAPS is a rare disease, randomized controlled trials have not been performed to guide and optimize treatment. The typical treatment approach for CAPS is a multidrug therapy that includes high-dose corticosteroids, intravenous immunoglobulin, and plasma exchange.13,14 In the present case, once the diagnostic suspicion was established, intervention with intravenous corticosteroids was begun immediately and maintained in conjunction with anticoagulation treatment. We did not have the availability to also undertake a prompt plasma exchange. Fortunately, the patient achieved a complete response. Following his discharge from the hospital, he did not develop new thrombotic events and maintained his clinical stability.
In conclusion, this case report describes an uncommon form of a rare syndrome (CAPS), confirmed by the Sapporo criteria plus preliminary classification criteria.1 Clinical suspicion based on the medical history and physical examination, but which could not be directly related to oligosymptomatic SARS-CoV-2 infection, led to consultation with physicians from various specialties and to the request and analysis of additional relevant clinical tests. As a result, the patient survived from a disease entity with typically high mortality.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consentInformed consent was obtained from the individual participant of the study.