A major complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT) is graft-versus-host disease (GVHD), an immune-mediated disorder that affects multiple tissues and organs with varying severity. Neurological complications (NC) of acute and chronic GVHD are rare but can produce severe clinical problems with significant morbidity and mortality.1,2
Based on the time of onset, NC are classified as early complications, usually related to conditioning regimen, bone marrow aplasia or drug toxicity; and late complications often related to GVHD and/or immunosuppressive therapy. They also can be divided into central or peripheral involvement.3 The reported incidence of NC after allo-HSCT varies widely, from 8% to 55%.4 NC with central nervous system involvement include stroke, seizures, headache and myelopathies.5
Peripheral Nervous system (PNS) complications are classified as single or multiple peripheral neuropathies, myopathy, or neuromuscular junction disorders.
Several mechanisms have been proposed to explain PNS involvement. The most common causes are related to drug toxicity, immune-mediated effects and complications of critical illness.4
Persistence or progression of a pre-existing neuromuscular disorder, sometimes undiagnosed, may also play a role in these patients.6
According to the consensus criteria of the National Institute of Health 2014, the diagnosis of any neurological manifestation of chronic GVHD requires the simultaneous existence of one typical diagnostic manifestation of chronic GVHD or at least one characteristic manifestation with a suitable additional test result.7,8
In the present study we report a case of alloimmune mediated multiple mononeuritis considered to be a GVHD after receiving stem cell transplantation, treated successfully with immunosuppressive therapy.
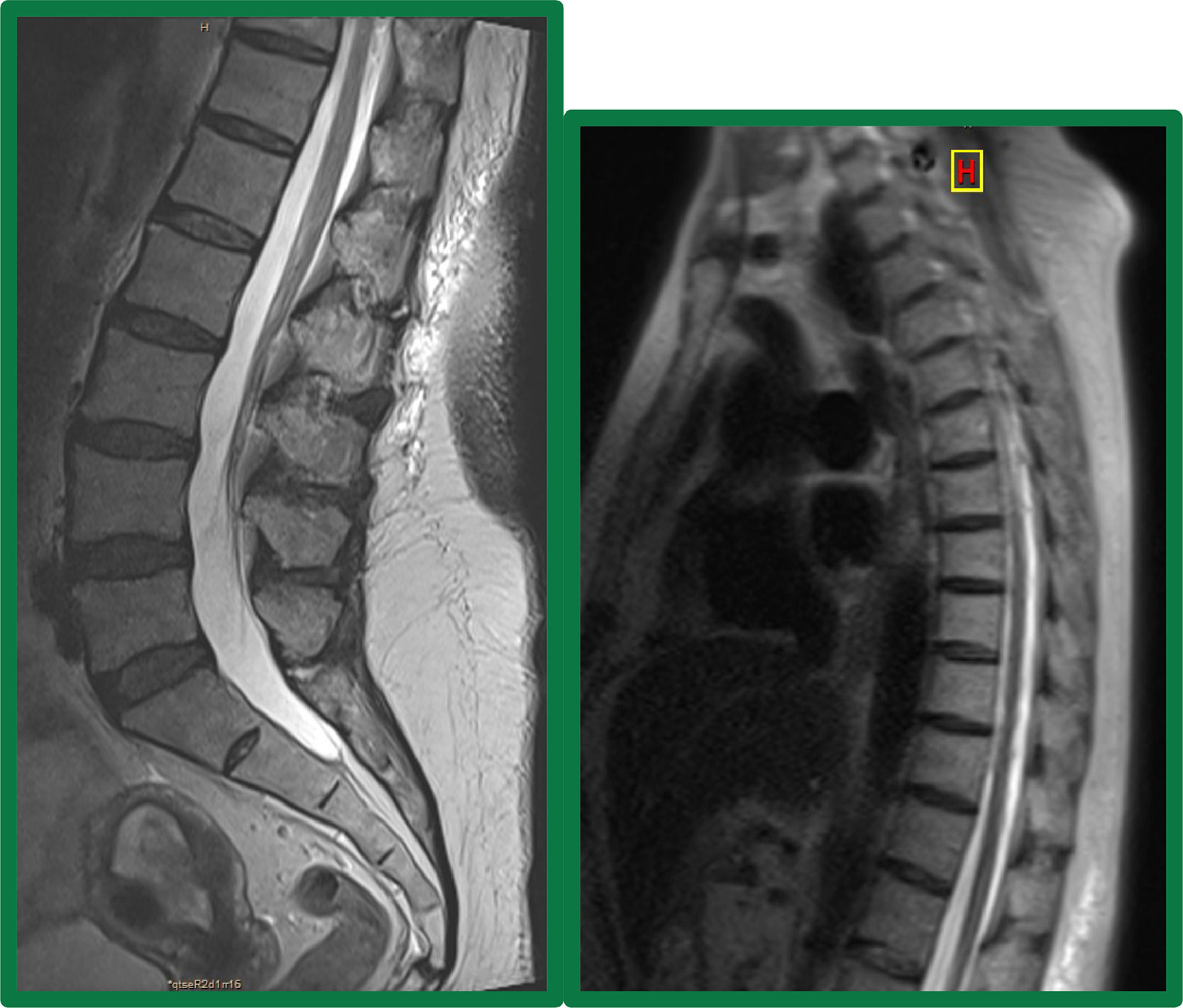
Case reportA 65-year-old female with a history of acute myeloid leukemia secondary to myelodysplastic syndrome was treated with intensive chemotherapy (3 plus 7 scheme with cytarabine and daunorubicin). As consolidation received a HLA-matched related allogeneic peripheral blood stem cell transplantation. Busulfan (3.2 mg/kg for two days) and fludarabine (25 mg/m2 for 5 days) were given as conditioning regimen and tacrolimus for GVHD prophylaxis. On day 90 after transplant, she was diagnosed with skin GVHD grade 2 on 18% of her body, receiving topical treatment and immunosuppressive treatment with tacrolimus with complete clinical resolution. Later, on day 180, she was admitted to the hospital due to low back pain.The patient complained of 7 days of persistent low back pain radiating to the flank and right thigh up to the knee. The physical examination showed predominantly proximal right femoral paresis, with tendon reflexes absent and preserved sensitivity. A magnetic resonance imaging (MRI) of the dorsal and lumbosacral spine was performed, and no alterations were found at the spinal cord level (Figure 1). The electromyogram revealed injury in the territory of the right crural nerve, which could correspond to an injury to the nerve itself or its L2-L3-L4 motor roots, with a severe deficit in the motor unit recruitment in the quadriceps and psoas muscles, without signs of denervation and with isolated signs of reinnervation.
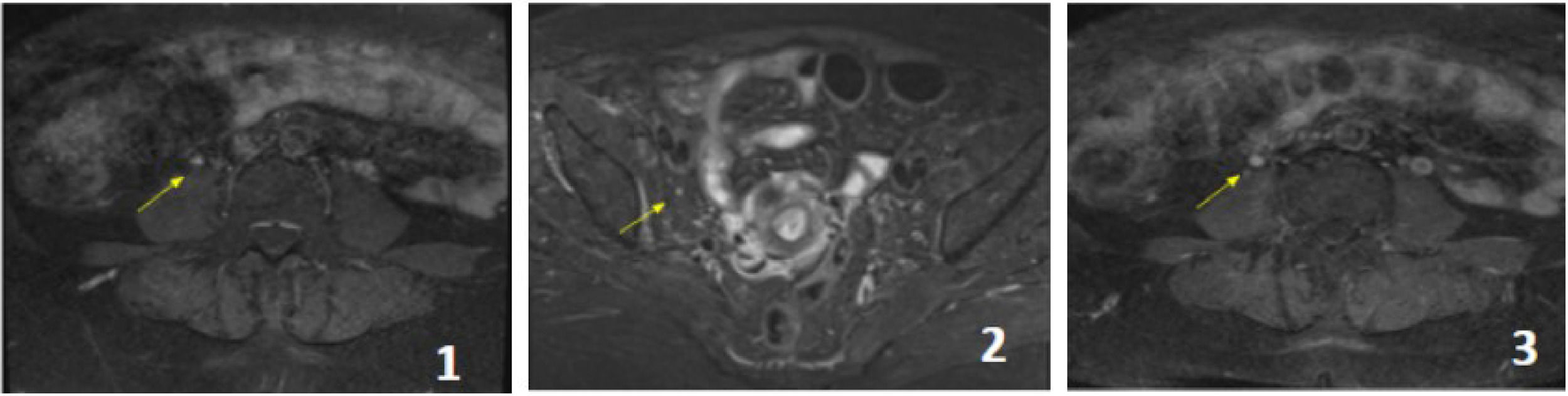
A new MRI with gadolinium showed a slightly increased signal intensity of the right femoral nerve in its abdominal and pelvic course on STIR sequence, with asymmetric post-contrast enhancement compared to its contralateral counterpart without signs of extrinsic compression; consistent with right femoral neuropathy (Figure 2).
Pelvic MRI with contrast, T1 sequences with contrast and STIR. The femoral nerve (yellow arrows) presents without signs of extrinsic compression. The signal is slightly increased in STIR sequence both in its abdominal path next to the psoas (1) and in its pelvic sector next to the iliac psoas (2). It presents enhancement after intravenous contrast administration, which is asymmetric with its contralateral counterpart (3).
Lumbar puncture was performed with normal cerebrospinal fluid (CSF) and negative polymerase chain reaction (PCR) for tuberculosis (TBC), cytomegalovirus (CMV), and varicella-zoster virus. CSF culture for TBC, bacteria, and fungi were negative, as well as VDRL in CSF, and serum CMV PCR. An autoimmune disease screening was performed with negative results.
To rule out a post-transplant lymphoproliferative syndrome (PTLD) and neurolymphomatosis, a 18 FDG PET/CT scan was performed, which did not show any abnormal FDG uptake.
A femoral biopsy was not feasible due to the risk of subsequent neurological sequelae and its difficult anatomical access. Based on these findings, a diagnosis of inflammatory mononeuropathy of immunological origin secondary to allo-HSCT was suspected.
The patient received empirical treatment with prednisone 1 mg / kg / day for 2 months and neuromuscular gait rehabilitation with complete recovery. Antimicrobial prophylaxis was prescribed according to local practice.
One month after finishing treatment, the patient presented with steppage gait and weakness in the dorsiflexion of the right foot. A new electromyogram showed right external popliteal sciatic nerve injury, with signs of denervation and lack of motor unit recruitment. A diagnosis of multiple mononeuritis of the same etiology was considered and the patient underwent a sural nerve biopsy to confirm the diagnosis. The pathology report showed preserved epineurium, perineurium and endoneurium, vasculitis with CD3 positive CD20 negative mononuclear infiltration, decreased myelin fiber density and axonal degeneration, compatible with immune mediated inflammatory neuropathy probably associated with chronic graft versus host disease.
Tacrolimus dose was increased and prednisone 1 mg/kg/day restarted. The patient also received gamma globulin 500 mg/kg/day for 5 days and neuromuscular training.
The patient made a full recovery again, but at 3 months while on steroid tapering, he presented a new event involving the external popliteal sciatic nerve. Increasing the steroid dose and the administration of gamma globulin continued to be effective in this case. However, to spare steroids and avoid new relapses, weekly 375 mg/m2 Rituximab for 4 doses was prescribed.
After twelve months of follow-up since the last dose of Rituximab, the patient's neurological status stabilized with complete recovery of the lesions of the right femoral sciatic nerve and the left external popliteal nerve. Nevertheless, persisted with mild motor sequelae in the right external popliteal sciatic nerve territory, with an inability to dorsiflex the foot. The patient has not presented new neurological events since then and is currently free of immunosuppressive treatment.
DiscussionImmune-mediated demyelinating diseases after allo-HSCT are rare and can occur with a frequency of 1–2%.9 In some cases, it is not entirely clear whether they are a true manifestation of chronic GVHD, an autoimmune disease in the context of a reconstitution of the immune system, a consequence of conditioning regimen, or an underlying infection.10 For this reason, they cannot be considered as hallmarks of chronic graft-versus-host disease.11 Patients usually present with a skin rash that precedes neuropathy after a reduction or discontinuation of immunosuppressive treatment.12 Patients may respond to the reintroduction of immunosuppressive drugs. Treatment with gamma globulin or plasmapheresis is usually not effective.6 Apart from acute inflammatory neuropathies, subacute or chronic inflammatory demyelinating polyradiculoneuropathy have been described in patients after allo-HSCT.13 As a variant, chronic immune-mediated axonal polyneuropathy following HSCT can occur.14 Cases of mononeuropathy have also been reported.3
It should be noted that there are also reported cases of immune-mediated demyelinating neuropathies without T-type lymphocytic inflammatory infiltrate at the nerve level and absence of manifestations compatible with chronic GVHD.15
The histology in these cases showed a high frequency of infiltration around the blood vessels, followed by activation or proliferation of microglia, demyelination, infiltration of CD3+/CD8+ T cells and in some cases perivascular infiltrating T cells were confirmed to be from donor sources.5
The most common cause of multiple mononeuropathies is vasculitic neuropathy (mononeuritis multiplex). Systemic and non-systemic forms exist. Systemic vasculitic neuropathy often occurs in the setting of longstanding rheumatologic conditions. It can also be seen with neoplasms, hereditary neuropathy.16
The patient's clinical characteristics met the diagnostic criteria for chronic GVHD: skin chronic GVHD was present at the time of the neurological manifestation and compatible pathology report. She also has steroid refractoriness and short gamma globulin response.
Treatment of steroid-refractory cGVHD patients with Rituximab, a B cell–depleting anti-CD20 monoclonal antibody, has shown a beneficial role in resolution of the autoimmune disorders and cGVHD, which was the reason for using it in our patient.17
In conclusion, early and late-onset of immune-mediated neurologic adverse events involving the peripheral or central nervous system may occur after allo-HSCT. The severity can range from a mild manifestation to life-threatening conditions. Patients with immune mediated neurologic manifestations benefit from early and intensive immunomodulatory treatment. All patients with neurological chronic GVHD manifestations should receive individualized, risk-adapted, and multidisciplinary care, so that any complications that arise can be correctly diagnosed and appropriately treated.18