Enteropathic-associated T-cell lymphoma (EATL) is a subtype of peripheral T-cell lymphoma (PTCL) which is aggressive in nature and usually affects the small intestines. The World Health Organisation (WHO) classifies EATL into 2 main types; the classical enteropathic-associated T-cell lymphoma Type 1(EATL-1) which is associated with celiac disease and the monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) which is often primary in nature.1 MEITL was formerly known as EATL Type 2 and it differs from EATL type 1 as to histomorphology and immunohistochemistry. MEITL is the most common primary T-cell lymphoma affecting the small bowel in Asia and is also frequently seen in Hispanics.2 Intestinal T-cell lymphoma which does not fulfil the criteria for either EATL type 1 or MEITL is often regarded as Intestinal T-cell lymphoma-not otherwise specified (ITL-NOS).
Case presentationA previously healthy 70-year-old female of Chinese ethnicity presented with a 4-week history of abdominal pain and persistent vomiting. She is a non-smoker, teetotaller, and a homemaker.
Physical examination revealed a febrile, cachexic and jaundiced female with stable vital parameters. Her abdomen was tender and mildly distended. There were no palpable lymphadenopathies or organomegaly.
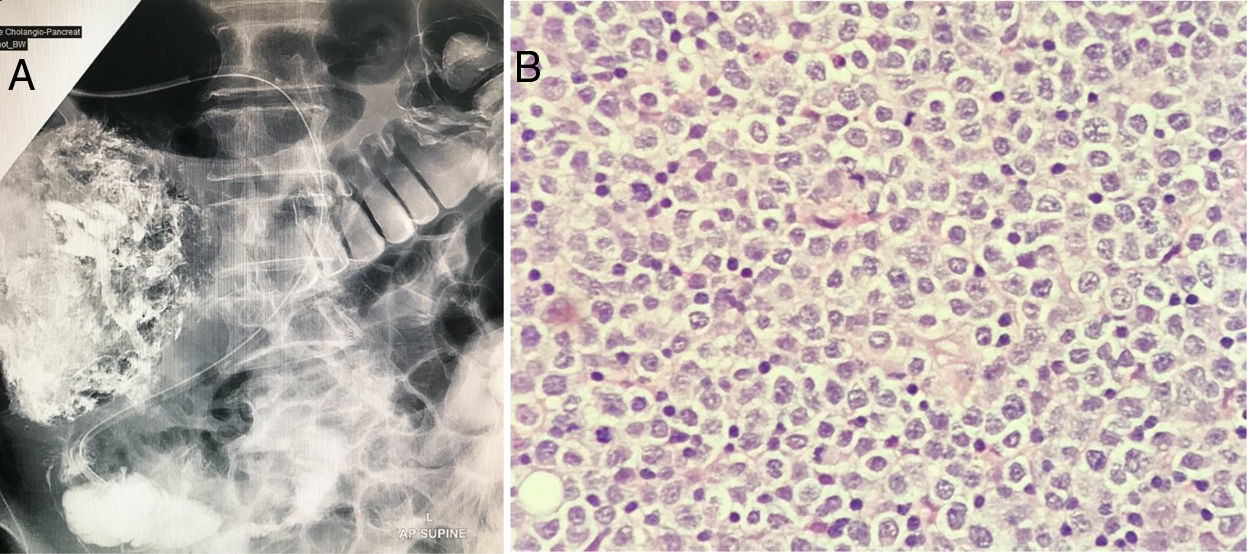
The blood parameters showed an increase of total bilirubin secondary to augmented direct bilirubin which resulted in obstructive jaundice. Other laboratory parameters are tabulated in Table 1. A whole-body Computed Tomography imaging was consistent with stage 1E disease (Ann Arbor staging system) which showed a mass of 3×4cm at the 2nd portion of the duodenum (D2) with no other lesions. In view of the obstructive jaundice, an endoscopic retrograde cholangiopancreatography (ERCP) was performed and it portrayed a duodenal mass associated with strictures and fibrosis (Fig. 1A). A gastrojejunostomy bypass was performed with the histology of the duodenal mass being consistent with MEITL. The neoplastic cells were described as monomorphic medium-sized lymphocytes, containing pale cytoplasm, round nuclei and exhibiting epitheliotropism (Fig. 1B). The tumour cells were positive for CD3, CD5, CD7, CD8, CD56, TCR gamma/delta, TIA-1 and granzyme B. They were negative for CD4, CD20, CD30 and Epstein Barr Virus encoded RNA (EBER). Ki67 proliferation index was 70%.
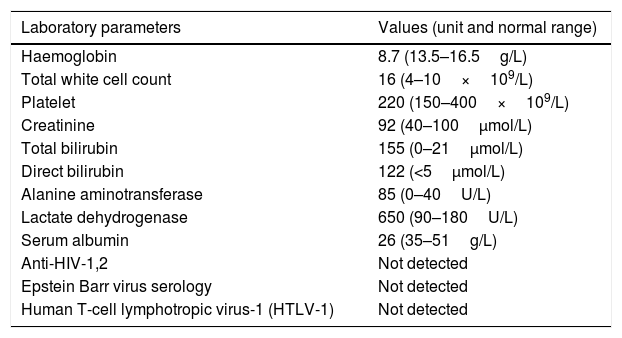
Tabulation of laboratory parameters.
| Laboratory parameters | Values (unit and normal range) |
|---|---|
| Haemoglobin | 8.7 (13.5–16.5g/L) |
| Total white cell count | 16 (4–10×109/L) |
| Platelet | 220 (150–400×109/L) |
| Creatinine | 92 (40–100μmol/L) |
| Total bilirubin | 155 (0–21μmol/L) |
| Direct bilirubin | 122 (<5μmol/L) |
| Alanine aminotransferase | 85 (0–40U/L) |
| Lactate dehydrogenase | 650 (90–180U/L) |
| Serum albumin | 26 (35–51g/L) |
| Anti-HIV-1,2 | Not detected |
| Epstein Barr virus serology | Not detected |
| Human T-cell lymphotropic virus-1 (HTLV-1) | Not detected |
She was treated with 2 cycles of anthracycline based chemotherapy, CHOP (cyclophosphamide 750mg/m2, doxorubicin 50mg/m2, vincristine 1.4mg/m2 and prednisolone 60mg/m2). She was able to tolerate the chemotherapy without any adverse effects. However, she had worsening abdominal symptoms such as vomiting and abdominal distension while on CHOP chemotherapy. She also had worsening anorexia and night sweats. A repeated abdominal CT imaging showed a progressive enlarging mass of 5×6cm at the 2nd portion of the duodenum (D2) with multiple new intra-abdominal lymphadenopathies. An oesophagogastroduodenoscopy (OGDS) was performed with the repeated duodenal mass biopsy being consistent with MEITL. She was then salvaged with 2 cycles of gemcitabine and platinum-based chemotherapy (gemcitabine 1000mg/m2, cisplatin 75mg/m2 and intravenous dexamethasome). However, she continued to have persistent abdominal symptoms and was increasingly cachexic. She subsequently developed upper gastrointestinal bleeding and succumbed to her illness within 4 weeks from the second chemotherapy salvage.
DiscussionMEITL is very rare and makes up less than 5% of all gastrointestinal lymphomas. MEITL is twice more common in males than in females. It disseminates and has a poor prognosis with a median survival of 7 months.3 There has been no published case reports or literature on MEITL in Malaysia. This case highlights the imperativeness of clinicopathological correlation in the diagnosis and management of MEITL in an elderly Asian female who presents with obstructive jaundice and abdominal distension. MEITL is often a forgotten diagnosis as many clinicians and histopathologists are unaware of such a disease.
The main differential diagnoses for primary gastrointestinal lymphomas are; duodenal-type follicular lymphoma (D-FL), NK/T-cell lymphoma (NK/TCL) and T-cell lymphoproliferative disorders (T-LPD). D-FL and T-LPD are usually low grade and indolent with a low Ki67.3 NK/TCL is frequently associated with Epstein Barr virus infection. Inflammatory disorders such as ulcerative colitis and lymphocytic colitis may also mimic the symptoms of MEITL.
Histomorphologically, the neoplastic cells in MEITL are described as monomorphic, small to medium-sized lymphocytes with pale cytoplasm, round nuclei and they exhibit epitheliotropism. They usually lack the inflammatory background which is frequently seen in the classical EATL type 1. The neoplastic cells are CD3+, CD4−, CD5−, CD8+, CD56+, CD30−, MATK+, EBER− and TCR-gamma delta+.4 Cytotoxic markers such as TIA-1, granzyme B and perforin are also present.4
There is no standardised therapy for MEITL. Most studies performed in Europe are tailored towards type 1 EATL and thus, the results cannot be extrapolated to MEITL. Furthermore, other studies available concentrate on PTCL in general without any specific mention on management of MEITL. However, regardless of EATL subtypes, both type 1 EATL and MEITL have a dismal prognosis. Treatment usually consists of a combination of surgery, chemotherapy, radiotherapy and autologous stem cell transplantation (ASCT). Anthracycline based polychemotherapy (cyclophosphamide, etoposide, vincristine and prednisolone) followed by up-front ASCT have been used with some improvement in overall survival.5 The Scotland and Newcastle Lymphoma group report has demonstrated significant higher progression free survival (PFS) and overall survival (OS) rates in patients receiving IVE/MTX polychemotherapy (ifosfamide, vincristine, etoposide/methotrexate) as compared to those who received anthracycline based chemotherapy.6 Moreover, some patients are elderly with poor functional status rendering them not suitable for ASCT. Other therapies include addition of alemtuzumab; an anti-CD52 monoclonal antibody. l-Asparaginase based regimens show a higher complete remission rate than CHOP or anthracycline based chemotherapy.7l-Asparaginase may be used as monotherapy in patients not fit for combination polychemotherapy. Anthracycline based chemotherapy such as CHOP chemotherapy is no longer used as standard regimens in other aggressive lymphomas such as NK/T cell lymphoma as it is frequently ineffective.8 The ineffectiveness of CHOP chemotherapy could be explained by the expression of CD56 on tumour cells and elevated presence of P-glycoprotein which are seen in both MEITL and NK/T Cell lymphoma.8 As MEITL is a rare disease, more prospective randomised multicentre studies are required to ascertain more information on molecular pathogenesis and effective combination polychemotherapy strategies.
ConclusionMEITL is a challenging primary intestinal T cell lymphoma to treat as the outcome is frequently poor despite surgery and chemotherapy. Most patients are elderly with co-morbidities and they usually present late rendering any therapy ineffective. Young age, early Ann-Arbor/Lugano disease stage, good performance scale status, patients receiving ASCT and less bulky disease are associated with an improved survival outcome. In-depth understanding of the molecular pathogenesis of the disease will give rise to more therapeutic approaches in the future.
Ethical approvalNot applicable as this is not a clinical trial.
Author's contributionThe author solely contributed towards the writing of this manuscript.
Funding statementSelf-funding.
Conflicts of interestThe author declares no conflicts of interest.
ConsentWritten informed consent was obtained from the next-of-kin for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
GuarantorGanesh Kasinathan is the guarantor of this manuscript.
None.