Children with Down syndrome (DS) present a higher risk for developing leukemia, with infants being especially prone to acute megakaryoblastic leukemia (AMKL).1 DS neonates are also predisposed to a reversible form of transient myeloproliferative disorder (TMD). Characteristically, AMKL and TMD are associated with mutations in the GATA1 gene, a transcription factor essential for erythroid and megakaryocyte differentiation. It is postulated that an increased RUNX1 gene expression due to constitutional trisomy 21 and somatic mutations in the GATA1 gene are the main mechanism processes responsible for the development of AMKL and TMD disorders.2
Hasle et al.3 showed that older children (>4 years old) with acute myeloid leukemia (AML) and DS have no mutation in the GATA1 gene, although they present diverse morphologic subtypes of AMKL, characterized as sporadic AML. In young children with DS, other AML subtypes such as minimally differentiated AML (AML-M0) are uncommon.2,3
AML-M0 represents only 5.3% of all AML cases in both Down and non-Down patients.4 Previous studies have emphasized that patients with AML-M0 have a high incidence of karyotypic abnormalities including hypodiploidy, complex karyotypes, –5/5q–, –7/7q, ins(10;11) and trisomy 8.4,5 Ring/marker chromosomes derived from chromosome 7 have been described in both DS and non-DS childhood AML.3,6
However, the GATA1 mutation may be considered a better marker of the unique DS-AMKL rather than age, blast count, French-American-British (FAB) classification type or cytogenetic aberrations.3
This work reports an uncommon case of a young child with DS-AML-M0 with monosomy 7, somatic ring chromosome 7 and no mutation of the GATA1 gene.
PatientA white 21-month-old boy was admitted to the Oncohematology Pediatric Center in Recife (CEONHPE), Brazil, with fever and dyspnea for six days and no previous hematological disease. His physical examination revealed irritability, bronchospasm, and hepatosplenomegaly. Laboratory tests showed a white blood cell count (WBC) of 10.6×109/L with 3% blasts, hemoglobin of 9.0g/dL and a platelet count of 10×109/L. The bone marrow was subsequently aspirated to investigate cytomorphologic and cytochemical aspects for FAB classification.3 Furthermore, immunophenotyping, cytogenetic and molecular studies were performed, as hereafter described in the methods.
Following the AML-M0 diagnosis, the child was treated for lower respiratory illness with a bronchodilator, antibiotics and blood transfusions and subsequently treated with the AML Berlin, Frankfurt, Munich (BFM) 2002 protocol modified for DS. After 12 days of chemotherapy, he presented fever and hemorrhagic suffusions (ecchymosis and petechiae), vomit and diarrhea. The child subsequently developed a septic stroke and was treated with antibiotics (voriconazole, imipenem/cilastatin, and vancomycin), as well as with platelet transfusions. However, he succumbed from pulmonary hemorrhage after eighteen days of treatment.
MethodsFlow cytometryImmunophenotyping was performed by flow cytometry after bone marrow aspiration following a Ficoll-Hypaque separation procedure; at least 20% of blasts were required to be considered positive. A routine panel of 27 antibodies was used: CD45, CD3, CD34, CD7, CD10, HLA-DR, CD19, sIgM, CD4, CD8, CD13, CD14, CD20, CD22, CD2, CD5, CD117, CD33, CD61, CD41, cCD79a, cIgM, cCD22, cCD3, aMPO and TdT.
Cytogenetic studiesCytogenetic analysis was performed of bone marrow according to standard protocols with G-banded karyotypes being characterized according to the International System for Human Cytogenetic Nomenclature (ISCN 2016).7
To investigate the ring chromosome, fluorescence in situ hybridization (FISH) experiments were carried out on metaphase spreads and interphase cells using a locus specific to chromosome 7 centromere probe according to the manufacturer's instructions. In addition, multicolor banding (MCB) probes for chromosome 7 and bacterial artificial chromosome (BAC) probes were analyzed for the regions 7p21.2 (RP11-79G16) and 7q34 (RP11-1141E10). The MCB probes were produced and labeled in the Molecular Cytogenetics Laboratory of the Institute of Human Genetics, in Jena, Germany. Moreover, BAC probes were selected from the University of California, Santa Cruz (UCSC) Genome Browser website (http://genome.ucsc.edu/) in February 2009 (GRCh37hg19) with clones having been obtained from an RP11 library. The BAC probes were cultivated in Luria-Bertani culture medium supplemented with chloramphenicol.8
GATA-1 investigationThe GATA-1 (exon 2) mutation was tested using genomic DNA from a peripheral blood sample. Direct sequencing was performed using primer sequences and a previously described method.9
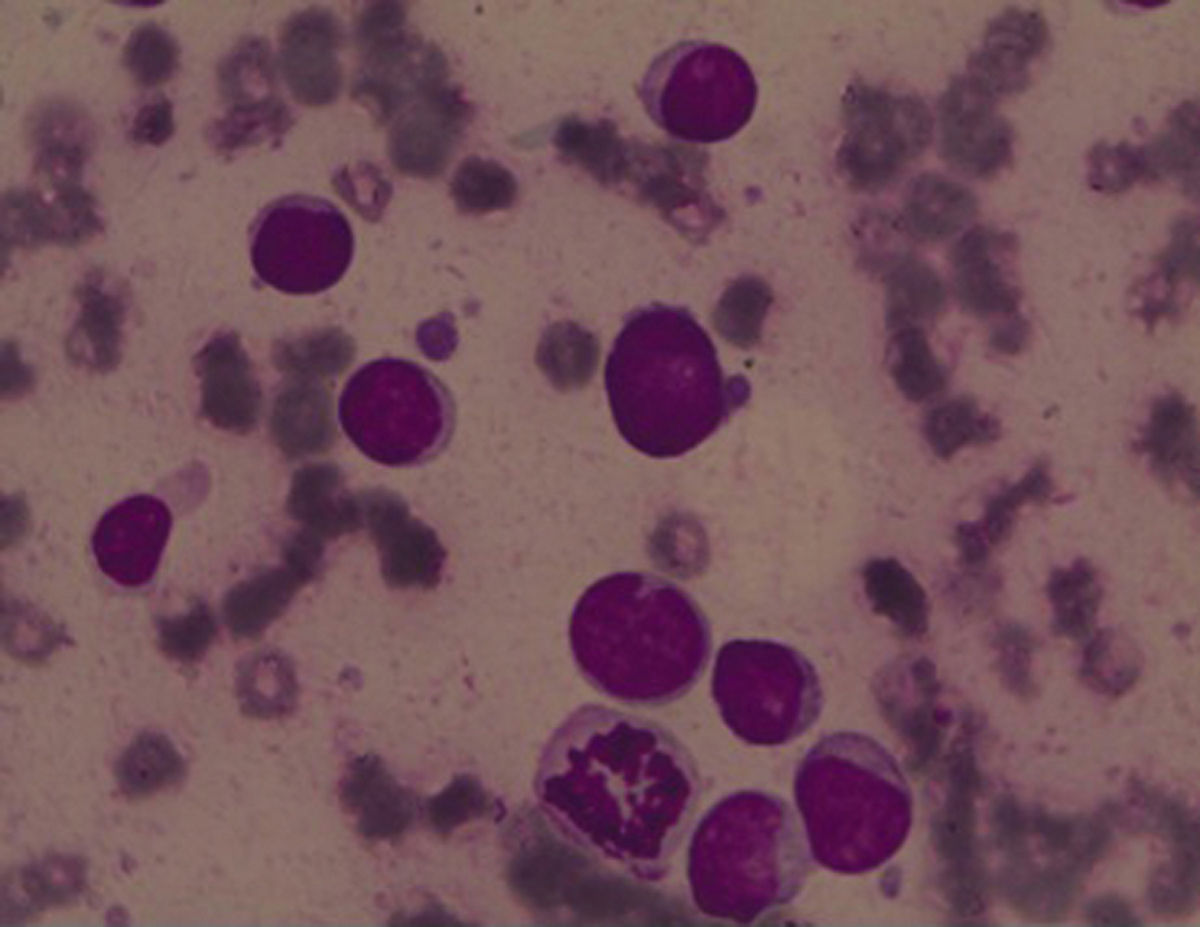
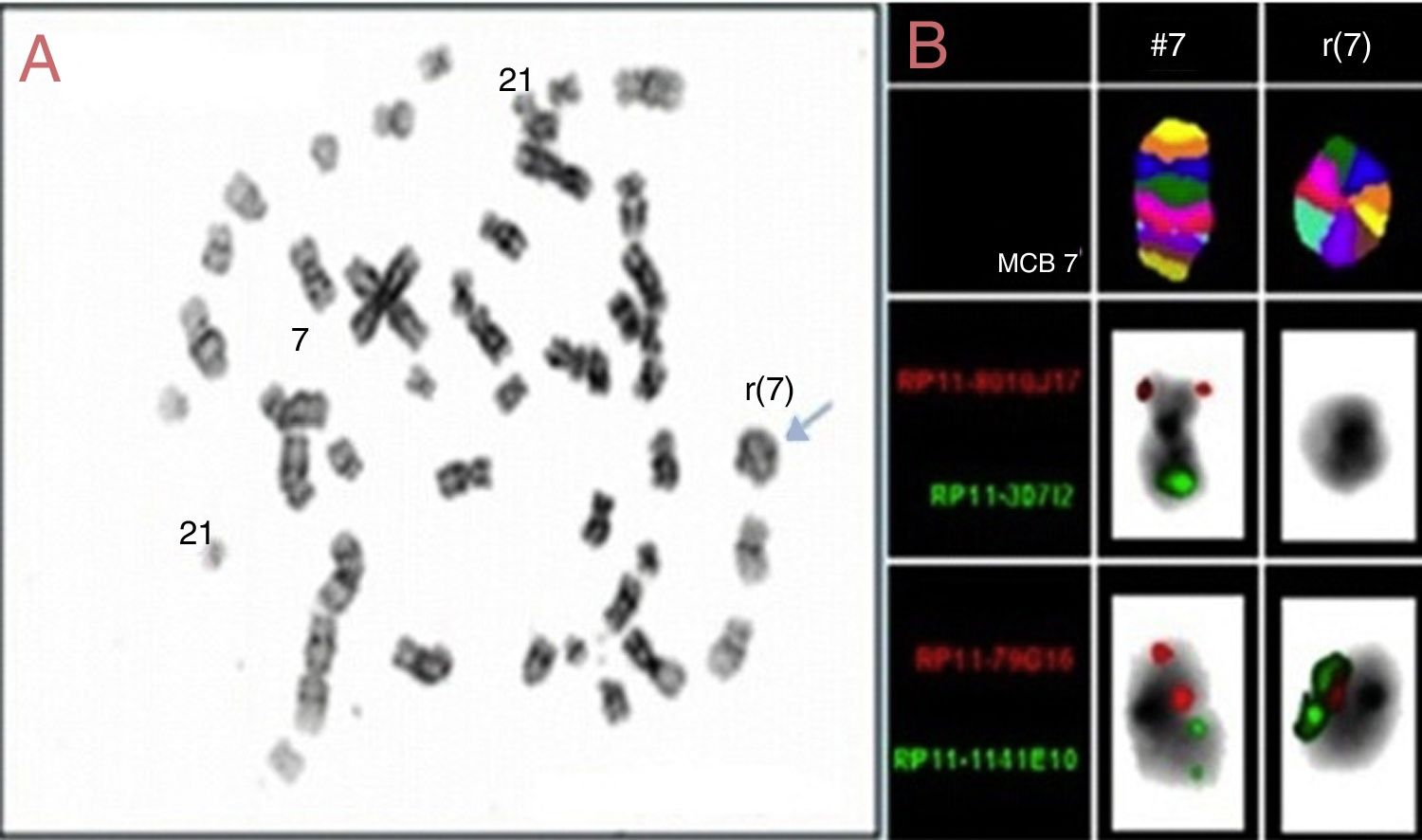
ResultsBone marrow aspiration presented 90% of undifferentiated blast cells (Figure 1) and Sudan black B (SBB) was negative. Flow cytometry analysis identified expressions of CD34, HLA-DR, CD117 and CD33 and was negative for MPO, CD41 and CD61. It was thus observed that the bone marrow karyotype was 47,XY,-7, +r, +21c7/15 (Figure 2A).
The locus-specific FISH probes identified two chromosomes, the MCB revealing a ring chromosome 7 and bacterial artificial chromosome probes showed breaks at 7p21.2 (RP11-79G16) and 7q34 (RP11-1141E10), characterizing the break and the junction of ring chromosome 7 (Figure 2B). Moreover, exons 2 and 3 of the GATA1 gene were not amplified by polymerase chain reaction, revealing a wild GATA1 gene.
DiscussionAlthough the importance of cytogenetic findings for the classification, prognosis and treatment in DS-AML cases has already been recognized, there is limited information available regarding clinical data, genetics, cytogenetic alterations and the prognosis in phenotypes other than AMKL, such as in the AML-M0 case described in this study.
AMKL in DS is believed to be a relatively homogeneous subgroup of leukemia with an excellent response to chemotherapy.2 However, it is unclear whether other DS-AML subgroups have the same behavior. Regardless of blast cell phenotype, monosomy, deletion, and rearrangement in chromosome 7 certainly play important roles in both pathogenesis and prognosis of AML. Cytogenetic abnormalities involving chromosome 7 found in this patient with DS-AML-M0, as in other reported cases, may either reflect an inherited cytogenetic instability or acquired genotoxic damage or even both. In DS, congenital GATA1 mutations provide helpful clues regarding the leukemogenesis in AMKL, although leukemogenic mechanisms remain unknown for other AML subtypes.4
Patients with AML, not uncommonly, have abnormalities of chromosome 7. Bunin et al.6 described three childhood AML cases with deletion or monosomy 7 (two cases of AMKL and one of AML-M0) and observed that the monosomy 7 in DS has a different significance from that in non-DS patients. However, according to Hasle et al.10 and Blink et al.,11 monosomy 7 and del(7q) are different in prognosis, both in DS and non-DS patients, with the correlation of monosomy 7 being considered a poor prognosis.
Fujino et al.12 described three cases of AMKL with ring 7 (one of DS and two non-DS) with good prognosis; another 14 AMKL cases have been described. However, the prognosis in DS-AML with abnormalities of chromosome 7, including ring chromosome 7, still need to be established. Furthermore, it is likely that the ring chromosome as well as del(7q) might differ from those with pure monosomy 7.
During the formation of ring 7 in the case described in this study, there was a deletion of the terminal region 7q35-qter before the ring junction in the 7p21 region. Loss of the 7q region is commonly associated with MDS, mainly in predisposing to leukemia syndrome, such as in DS, Fanconi anemia and neurofibromatosis.6 In these cases, breaks in the 7q region mainly occur between 7q11 and 7q36. These regions shelter important genes, such as EHZ2, a component of the polycomb repressive complex 2 (PRC2), localized in the 7q36.1 region. This component participates in DNA methylation, and it has recently been described as transcriptional repression related to diverse types of cancer.13
Ring chromosome formation may occur through breaks in the chromosome arms and fusion of the proximal broken ends, leading to the loss of distal material. In addition, rings may be formed by telomere dysfunction that triggers fusion of the reactive chromosome ends without a major loss of genetic material.14
The authors of this study believe that this is the first reported case of AML-M0 in DS defined by molecular cytogenetic, MCB and BACs that shows lost regions and a new junction in ring chromosome 7. This reported case occurred in an infant with DS, though the biologic characteristics were of an older sporadic DS-AML without the GATA-1 mutation. Nonetheless, a low number of blast cells in the sample or even the AML-M0 could be accountable for this result. Although this case had a poor outcome, few cases of AML M0 in DS have been described to draw any conclusions.
FundingSupported by Fundacão de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE), CAPES (PROBAL//DAAD—Project no. 419/14), Brazil-Germany; CNPq, Brazil.
Conflicts of interestThe authors declare no conflicts of interest
We are grateful to Dr. Maria S. Pombo-de-Oliveira that kindly provided the GATA-1 sequencing test, performed at the Pediatric Hematology and Oncology Program Research Center, Instituto Nacional de Câncer, Rio de Janeiro.