This study investigated the association of Robin Sequence with ABO and RhD blood group phenotypes.
MethodsA retrospective cross-sectional study was performed of a cohort of Robin Sequence patients of the Hospital de Reabilitação de Anomalias Craniofaciais – Universidade de São Paulo (USP), Brazil. The study group was composed of 339 individuals of both genders with Robin Sequence referred for specific treatment. A control group was composed of 1780 individuals without syndromes. The groups were compared using the Pearson’ chi-square test (χ2) with statistical significance being defined for an alpha error of 5% (p-value<0.05).
ResultsA comparison of gender found a significant difference for the AB phenotype between groups (p-value=0.007). Comparing blood type by gender there was no significant difference within the same group (p-value=0.117 and 0.388 respectively, for Robin Sequence and the control group). When comparing the AB blood type between groups, there was no difference for females (p-value=0.577), but there was a significant difference for males (p-value=0.0029).
ConclusionsThis study showed that the population with Robin Sequence had different patterns related to gender concerning the phenotypic distribution of ABO and RhD blood group phenotypes. Robin Sequence is more common among females. The AB phenotype was significantly higher in males with Robin Sequence than in males of the Control Group. The prevalence of the RhD-negative phenotype is higher in individuals with Robin Sequence. This result suggests a possible association of ABO and RhD phenotypes with Robin Sequence that should be better investigated by molecular studies, as it deserves greater attention.
The ABO and RhD blood group phenotypes are classified by antigens expressed in the erythrocyte glycocalyx and defined by genetic expression.1–3 The gene encoding of ABO blood group phenotypes is located on the long arm of chromosome 9 and is responsible for a type II protein, a singular amine- or carboxy-terminal plasma transmembrane molecule, which expresses four antigens (A, B, AB and O).1,2,4–6 The RHD and RHCE genes, located on the short arm of chromosome 1, encode Rh antigens.3–5,7
The study conducted by Ponce and Valverde demonstrated a relationship between the expression of ABO antigens and the helminth Ascaris lumbricoides, revealing that individuals with the A phenotype are at higher risk of infestation by this parasite.8 In 2012, a study by Mäkivuokko et al. demonstrated that individuals with the B phenotype present a greater number of the bacterium Clostridium leptum in the bowel lumen.9 Storry and Olsson demonstrated a correlation between Plasmodium vivax and the absence of the AB phenotype in individuals of African descent, who present suppression of alleles of the ABO genes causing an absence of the receptor for the malaria parasite in erythrocytes.10
The Robin Sequence (RS) is an autosomal recessive craniofacial malformation with intense phenotypic expression, considered a sequential pathogenesis11 or a non-specific symptomatic complex, which may occur in several situations: in isolation, (isolated RS) or associated with known syndromes including the Stickler, Treacher-Collins and Van der Woude syndromes, or in association with other developmental defects that do not constitute a specific syndrome. The main characteristic of RS is a triad of anomalies: micrognathia (mandibular hypodevelopment), glossoptosis (posterior tongue displacement) and cleft palate causing difficulties in sucking and swallowing that can cause obstruction of the airway and even death in some cases.12–15
The study of Jakobsen et al. of a human cytogenetic database, identified putative chromosomes and genes, 4q32-qter, GAD67 (2q31), PVRL1 (11q23-q24), and the SOX9 gene (17q24.3-q25.1),16 with the latter also being described by Selvi & Mukunda17 and other authors as playing an important role in RS.18–20
Considering the importance of genetic interactions in blood groups and the lack of studies applied to populations of individuals with RS, this study investigated the association of RS with ABO and RhD blood group phenotypes.
MethodsThis study was approved by the Institutional Review Board of the Hospital de Reabilitação de Anomalias Craniofaciais, Universidade de São Paulo (USP) (protocol #665.580). All data of ABO and RhD blood group phenotypes were obtained from secondary sources such as the patients’ medical records. The test is mandatory before any surgical procedure in the institution.
The study group included 339 individuals of both genders with RS registered at the institution. A control group was composed of 1780 individuals without any syndromes, attending appointments in the Ear, Nose and Throat Department. All individuals were Caucasians of Brazilian nationality.
The groups were compared using the Pearson chi-square test with statistical significance being defined for an alpha error of 5% (p-value <0.05).
ResultsAmong the 339 patients with RS, 45% (153/339) were males and 55% (186/339) were females. Of the 1780 individuals in the control group, 51% (909/1780) were males and 49% (871/1780) were females.
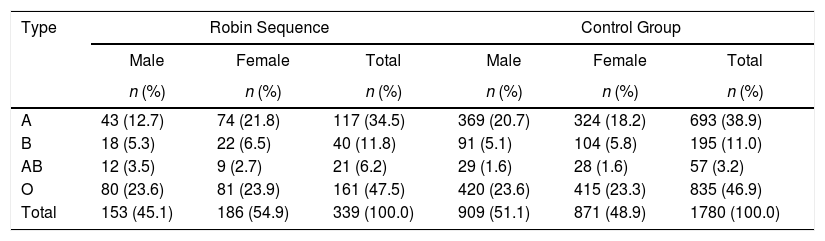
Table 1 shows the comparison between the RS and control groups according to the ABO phenotype. The data revealed a predominance of the O phenotype (47.5% vs. 46.9%), followed by A (34.5% vs. 38.9%), B (11.8% vs. 11%) and AB (6.2% vs. 3.2%), respectively. A comparison of gender found a significant difference for the AB phenotype between groups (p-value=0.007).
Comparison between the Robin Sequence and control group according to the ABO phenotype.
| Type | Robin Sequence | Control Group | ||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| A | 43 (12.7) | 74 (21.8) | 117 (34.5) | 369 (20.7) | 324 (18.2) | 693 (38.9) |
| B | 18 (5.3) | 22 (6.5) | 40 (11.8) | 91 (5.1) | 104 (5.8) | 195 (11.0) |
| AB | 12 (3.5) | 9 (2.7) | 21 (6.2) | 29 (1.6) | 28 (1.6) | 57 (3.2) |
| O | 80 (23.6) | 81 (23.9) | 161 (47.5) | 420 (23.6) | 415 (23.3) | 835 (46.9) |
| Total | 153 (45.1) | 186 (54.9) | 339 (100.0) | 909 (51.1) | 871 (48.9) | 1780 (100.0) |
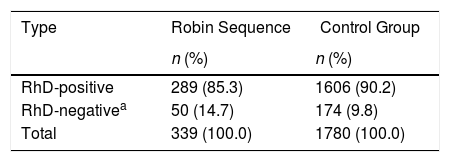
Table 2 shows the comparison between the RS and control groups according to the RhD phenotype. Analysis of the data of both groups showed a predominance of RhD+ (85.3% and 90.2%). Comparison between the RS group and the control group according to the RhD phenotype and genders revealed no significant differences.
Comparison between control group and Robin Sequence according to the RhD phenotype.
| Type | Robin Sequence | Control Group |
|---|---|---|
| n (%) | n (%) | |
| RhD-positive | 289 (85.3) | 1606 (90.2) |
| RhD-negativea | 50 (14.7) | 174 (9.8) |
| Total | 339 (100.0) | 1780 (100.0) |
Craniofacial anomalies, such as cleft lip, cleft palate and RS, are caused by changes occurring during human embryonic development and may be observed in the embryo in the early days of gestation.11,21
The main characteristic of RS is a triad of anomalies comprising micrognathia (mandibular hypodevelopment), glossoptosis (posterior tongue displacement causing pharyngeal obstruction) and cleft palate in nearly 90% of cases of RS.13,14
The clinical presentation of individuals with RS varies widely. Due to these changes, especially in the neonatal period, the child may have problems such as feeding difficulties and breathing disorders, with the possibility of respiratory obstruction or even severe crises of asphyxia, which may lead to death if not promptly managed.12–14
The association of ABO and RhD blood group phenotypes with different diseases is receiving more attention from the scientific community. Studies by Greer et al. demonstrated that individuals with the O phenotype present a significant association with the development of pancreatic cancer.22 Harris et al. evidenced that individuals with the O phenotype were more likely to develop symptomatic disease if infected with Víbrio cholerae.23 Tamega et al. revealed predominance of the A phenotype in individuals with chronic discoid lupus erythematosus, thereby facilitating the prognosis in disseminated clinical presentations of the disease.24 The study of Sharma et al. demonstrated a higher prevalence of malocclusions in individuals with the B phenotype in the population of Jaipur, India.25 It is important to point out that the present research demonstrated a possible association of RS with ABO and RhD blood group phenotypes. Therefore, it is necessary to investigate other phenotypes and respective genotypes for possible imbalances in the linkage in order to understand the causes of this craniofacial malformation.
Regarding gender, there was prevalence of 55% of females in individuals with RS, thereby corroborating the literature. Studies conducted by Meyer et al. revealed 64.2% of females among individuals with RS.26 Schaefer et al. analyzed 21 individuals with RS and observed 66.7% of females27 and Neto et al. found 75% of females.28 Therefore, both the present results and previous findings in the literature demonstrated a higher prevalence of females compared to males in non-syndromic RS patients.
Among the ABO phenotypes, there was predominance of the O phenotype, followed by A, B and AB in a population with RS, corroborating the results of Batissoco et al. and Barbosa et al.1,29 However, in the present study, a comparison of gender found a significant difference for the AB phenotype between groups (p-value=0.007).
Comparing phenotype and gender in the same group, there were no significant differences between the RS and GC Groups. Furthermore, on comparing gender between groups, there was no significant difference for females, yet there were more males in the RS Group than in the Control Group (p-value=0.0029).
Even though this study revealed a high frequency of RS individuals with the RhD negative phenotype, it is not possible to conclude that this erythrocyte antigen directly or indirectly influences the malformation; however, the study of Beiguelman revealed that the RhD-negative phenotype is prevalent in 15% of the world population.4
The Hospital for Rehabilitation of Craniofacial Anomalies is a nationwide reference center for rehabilitation of individuals with craniofacial anomalies and therefore assists individuals from many different ethnical backgrounds. However, within the scope of orofacial clefts and anomalies, there is predominance of Caucasians of Brazilian nationality.30
ConclusionThis study was limited to obtaining data from secondary sources, namely from the patients’ medical records. The study concluded that, originally, the population with RS had a different pattern related to gender concerning the phenotypic distribution of ABO and RhD blood group phenotypes. The RS is more common among females. Phenotype AB was significantly more prevalent in male individuals with RS. The prevalence of the RhD-negative phenotype is higher in individuals with RS. Therefore, this result suggests a possible association of ABO and RhD blood group phenotypes with RS. Further studies should be carried out to elucidate possible interactions between genotypes and phenotypes of other erythrocyte antigens besides ABO and RhD in individuals with RS.
This study was supported by the Hospital for Rehabilitation of Craniofacial Anomalies, Universidade de São Paulo (USP), Brazil. Collaboration with statistical data by Ms Flávia Maria Ravagnani Neves Cintra and Hilton Coimbra Borgo PhD.
Conflicts of interestThe authors declare no conflicts of interest.