Folate deficiency (FD) is a common concern in the clinical practice, particularly when investigating patients with macrocytic anemia. The importance of folate has been recognized in women of childbearing age since the 1980s due to its ability to prevent neural tube defects.1,2 Adequate use and accurate interpretation of the available laboratory tests used to diagnose this nutritional deficiency are necessary, and folate food fortification (FFF) adds yet another variable to this equation.
In the current issue of Hematology, Transfusion and Cell Therapy, Godoy and Tabares3 provide interesting retrospective data on the epidemiology of FD in a tertiary care hospital in Argentina, before and after folic acid wheat flour supplementation was implemented. Their main findings support that FD, as defined by a serum folate lower than 3ng/mL (6.7nmol/L), became a very rare condition after FFF, with a yearly incidence of 1.7%. In addition, the authors also draw attention to another fact: low serum folate levels were treated with supplementation in only 55% of cases implicating that providers will often overlook this diagnosis, even when confirmed by a blood test.
This Argentinean research adds to the bulk of evidence from studies that show FFF is a successful strategy against FD in public health and suggests that measuring serum folate is of very little, if any, clinical utility in populations exposed to FFF. This is a fair suggestion, even more so if physicians will not act on it when abnormal.
But kicking the habit of measuring serum folate, especially when investigating macrocytic anemia, is hard. Many of us find comfort in our (incorrect) conviction that, for instance, “if the serum folate is normal, I can rule out folate deficiency”. While a low serum folate supports this diagnosis, a normal level can be found in patients with recent adequate folate intake, even if only in small amounts in fortified food. That means FFF decreases the sensitivity of the serum folate test to detect FD, in which case red blood cell folate would be more informative. Testing for folate in erythrocytes comes at much higher complexity and cost and the results can be affected by cobalamin deficiency. The realization that blood folate is of low usefulness supports the potential saving of up to hundreds of thousands of dollars per year if red blood cell folate tests are discontinued in certain healthcare settings.4,5 It has been recommended that measuring homocysteine, a nonspecific functional biomarker of FD, is a more sensitive way of detecting FD than assessing blood folate.6 It is worth remembering that hyperhomocysteinemia is also present in cobalamin deficiency, so a correct interpretation of homocysteine comes at the expense of concurrently measuring vitamin B12, or rather, its associated metabolite, methylmalonic acid, whose levels will be increased even in the presence of borderline cobalamin levels. A comprehensive review of folate biomarkers and their caveats can be found elsewhere.7
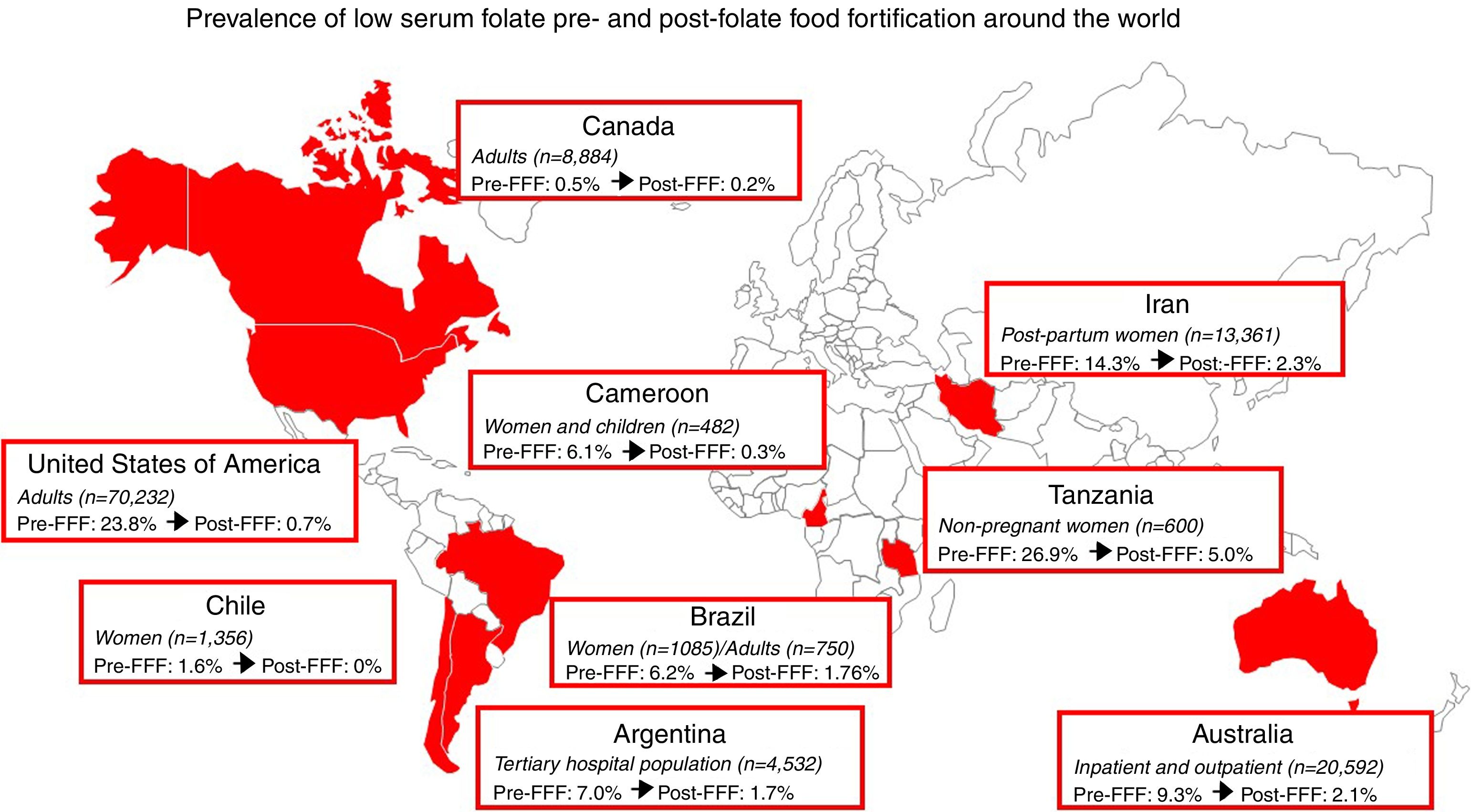
Ranging from 15.5 to 59% across different countries around the globe, the success of FFF in reducing the incidence of neural tube defects is undeniable.8,9Fig. 1 highlights that over 120000 individuals have been included in studies in nine different countries across four continents to demonstrate that the prevalence of FD, as determined by low serum folate levels, after the implementation of a mandatory FFF program, is about 2% or lower. The exception to this is the Tanzanian study,10 with a final prevalence of 5%, but with a very significant drop from 26.9%, and that after only 12 months of FFF. Data vary depending on the cutoff value used to define FD, which was 3ng/mL (6.7–7nmol/L) in all but the Tanzanian study, which used 4ng/mL (or about 9nmol/L), thus potentially overestimating the prevalence of FD. The paucity of studies investigating the value of folate measurements in populations more likely to have this nutritional deficiency, such as patients investigating macrocytic anemia, makes us wonder: should we still measure serum folate in countries with FFF?
Prevalence of FD in different populations, as determined by serum folate levels, before and after mandatory FFF programs around the world. Data represent the studies with the largest populations in each country (Argentina,3 Australia,11 Brazil,12,13 Cameroon,14 Canada,15 Chile,16 Iran,17 Tanzania,10 and the United States of America18).
One should remember that the positive predictive value of a test decreases with the prevalence of the disorder being tested for, so FFF decreases the chances of any folate biomarker being abnormal. Therefore, history taking should focus on identifying which patients are more likely to benefit from getting tested for folate, i.e. those with conditions which obliviate the effect of FFF. These include inadequate intake (malnutrition, low intake of fortified food, alcoholics), folate malabsorption (celiac disease, inflammatory bowel disease, tropical sprue, bariatric surgery, or secondary to certain medications, e.g. metformin, anticonvulsants, triamterene, etc.), and the rare carriers of mutations in the proton-coupled folate transporter gene SLC46A1.
While more studies on the adequacy of folate testing may be useful in specific populations, physicians in countries with FFF programs should take this into consideration and may be able to improve the cost-effectiveness of their diagnostic investigation by reducing folate testing to a minimum. Most important of all, if physicians eventually choose to test their patients for folate biomarkers, they should ensure that abnormal results are checked and patients adequately treated. After all, testing positive for FD and not getting treatment is unthinkable.
Conflicts of interestThe author has no conflicts of interest.
See paper by Ferreira MG et al. on pages 305–309.