Myelodysplastic syndromes (MDS) are myeloid neoplasms characterized by elevated rates of intramedullary apoptosis, associated with cytopenias in peripheral blood and with an increased risk of progression into acute myeloid leukemia (AML). The clinical course is highly heterogeneous, and different prognostic models have been designed to stratify patients into groups according to risk of disease progression and death.1,2 The only current curative treatment available is hematopoietic stem cell transplantation; however, the high treatment-associated morbimortality demands a need for the development of targeted therapies. Interleukin Receptor Associated Kinase-1 (IRAK1) is a protein that has intrinsic Toll-like Receptor/Interleukin-1 Receptor (TLR/IL-1R)-induced kinase activity. IRAK1 binds and activates TNF receptor-associated factor 6 (TRAF6) and induces signaling through nuclear factor-κB (NF-κB).3 IRAK1 and TRAF6 are negatively regulated by miR-146a, which is frequently expressed at low levels in MDS.4,5 Rhyasen et al.6 described that the IRAK1 protein is overexpressed and highly phosphorylated in MDS patients. IRAK1 pharmacologic inhibition resulted in reduced cell proliferation in vitro and in amelioration of anemia in a human xenograft mouse model. Also, IRAK1 mRNA was overexpressed in CD34+ bone marrow cells from MDS patients, and high IRAK1 expression correlated with reduced overall survival in a cohort of 20 MDS patients.6
In order to further investigate the role of IRAK1 mRNA expression as a potential prognostic marker in MDS, our aim was to characterize IRAK1 mRNA expression in a cohort of Brazilian MDS patients and to verify whether IRAK1 expression associates with clinical and laboratory features or impacts on event-free survival (EFS) and overall survival (OS) in MDS.
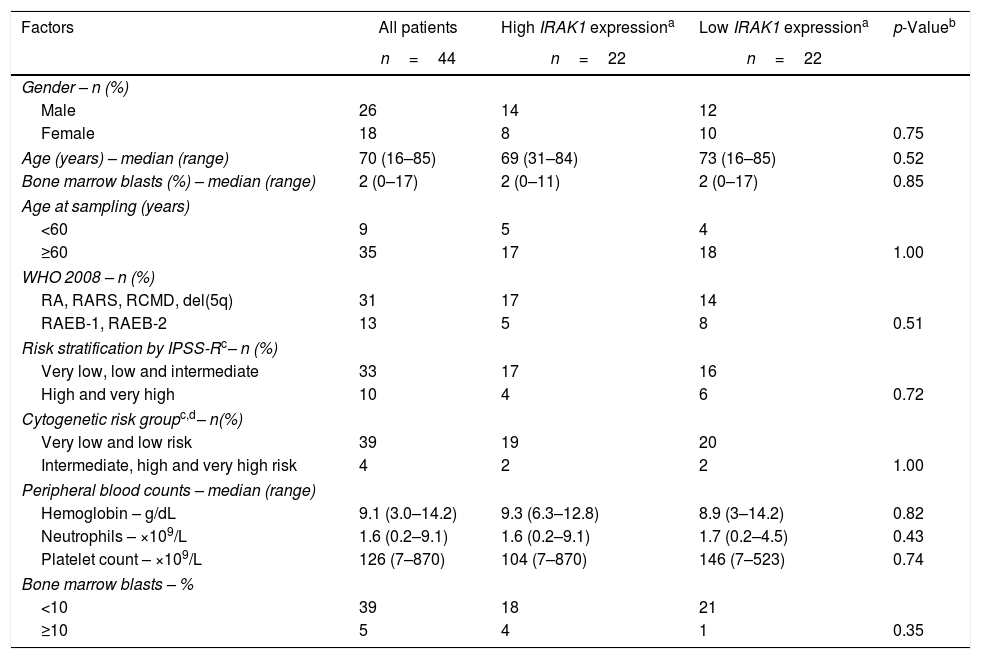
Bone marrow samples were collected from 14 healthy donors and 44 MDS patients followed up at the Hematology and Hemotherapy Center of the Universidade Estadual de Campinas between 2005 and 2013. Diagnosis was confirmed independently by three experts. Patients were untreated at the time of sample collection. The Institutional Review Board approved this research and all subjects provided informed written consent. For the purpose of this study, patients were classified according to the World Health Organization (WHO) 2008 classification1 and the revised international prognostic scoring system (IPSS-R).7 Patients’ characteristics are shown in Table 1.
Clinical and laboratory features of myelodysplastic syndrome patients stratified according to IRAK1 expression.
| Factors | All patients | High IRAK1 expressiona | Low IRAK1 expressiona | p-Valueb |
|---|---|---|---|---|
| n=44 | n=22 | n=22 | ||
| Gender – n (%) | ||||
| Male | 26 | 14 | 12 | |
| Female | 18 | 8 | 10 | 0.75 |
| Age (years) – median (range) | 70 (16–85) | 69 (31–84) | 73 (16–85) | 0.52 |
| Bone marrow blasts (%) – median (range) | 2 (0–17) | 2 (0–11) | 2 (0–17) | 0.85 |
| Age at sampling (years) | ||||
| <60 | 9 | 5 | 4 | |
| ≥60 | 35 | 17 | 18 | 1.00 |
| WHO 2008 – n (%) | ||||
| RA, RARS, RCMD, del(5q) | 31 | 17 | 14 | |
| RAEB-1, RAEB-2 | 13 | 5 | 8 | 0.51 |
| Risk stratification by IPSS-Rc– n (%) | ||||
| Very low, low and intermediate | 33 | 17 | 16 | |
| High and very high | 10 | 4 | 6 | 0.72 |
| Cytogenetic risk groupc,d– n(%) | ||||
| Very low and low risk | 39 | 19 | 20 | |
| Intermediate, high and very high risk | 4 | 2 | 2 | 1.00 |
| Peripheral blood counts – median (range) | ||||
| Hemoglobin – g/dL | 9.1 (3.0–14.2) | 9.3 (6.3–12.8) | 8.9 (3–14.2) | 0.82 |
| Neutrophils – ×109/L | 1.6 (0.2–9.1) | 1.6 (0.2–9.1) | 1.7 (0.2–4.5) | 0.43 |
| Platelet count – ×109/L | 126 (7–870) | 104 (7–870) | 146 (7–523) | 0.74 |
| Bone marrow blasts – % | ||||
| <10 | 39 | 18 | 21 | |
| ≥10 | 5 | 4 | 1 | 0.35 |
WHO: World Health Organization; RA: refractory anemia; RARS: refractory anemia with ringed sideroblasts; RCMD: refractory cytopenia with multilineage dysplasia; del(5q): myelodysplastic syndromes with isolated del(5q); RAEB-1: refractory anemia with excess blast-1; RAEB-2: refractory anemia with excess blast-2; IPSS-R: revised international prognostic scoring system.
RNA was extracted from total bone marrow cells using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). The reverse transcription reaction was performed using the RevertAid™ First Strand cDNA Synthesis Kit (MBI Fermentas, St. Leon-Rot, Germany). Expression of IRAK1 mRNA was detected by Maxima Sybr Green qPCR master mix (MBI Fermentas, St. Leon-Rot, Germany) in an ABI 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using specific primers for IRAK1 (FW: CGGTGTATGCTGTGAAGAGG; RV: AGCCGTTCTGAGCACAGTAG) and HPRT1 (FW: GAACGTCTTGCTCGAGATGTGA; RV: TCCAGCAGGTCAGCAAAGAAT). HPRT1 was used as the reference gene. The relative quantification value was calculated using the equation 2−ΔΔCT.8 A negative ‘no template control’ was included for each primer pair. The dissociation protocol was performed at the end of each run to check for non-specific amplification. Three replicas were run on the same plate for each sample. Statistical analyses were performed using Stata version 14.1 (Stata Corporation, College Station, TX, USA). For comparisons, Fisher's exact test was used for categorical factors with two levels; the Mann–Whitney test was used for measured factors, as appropriate. Cox regression model was used to estimate the EFS and OS. EFS was defined as the time of sampling to the date of progression to high-risk MDS or AML with MDS related changes, date of death or last follow-up, and OS was defined from the time of sampling to date of death or last follow-up. A p-value<0.05 was considered statistically significant.
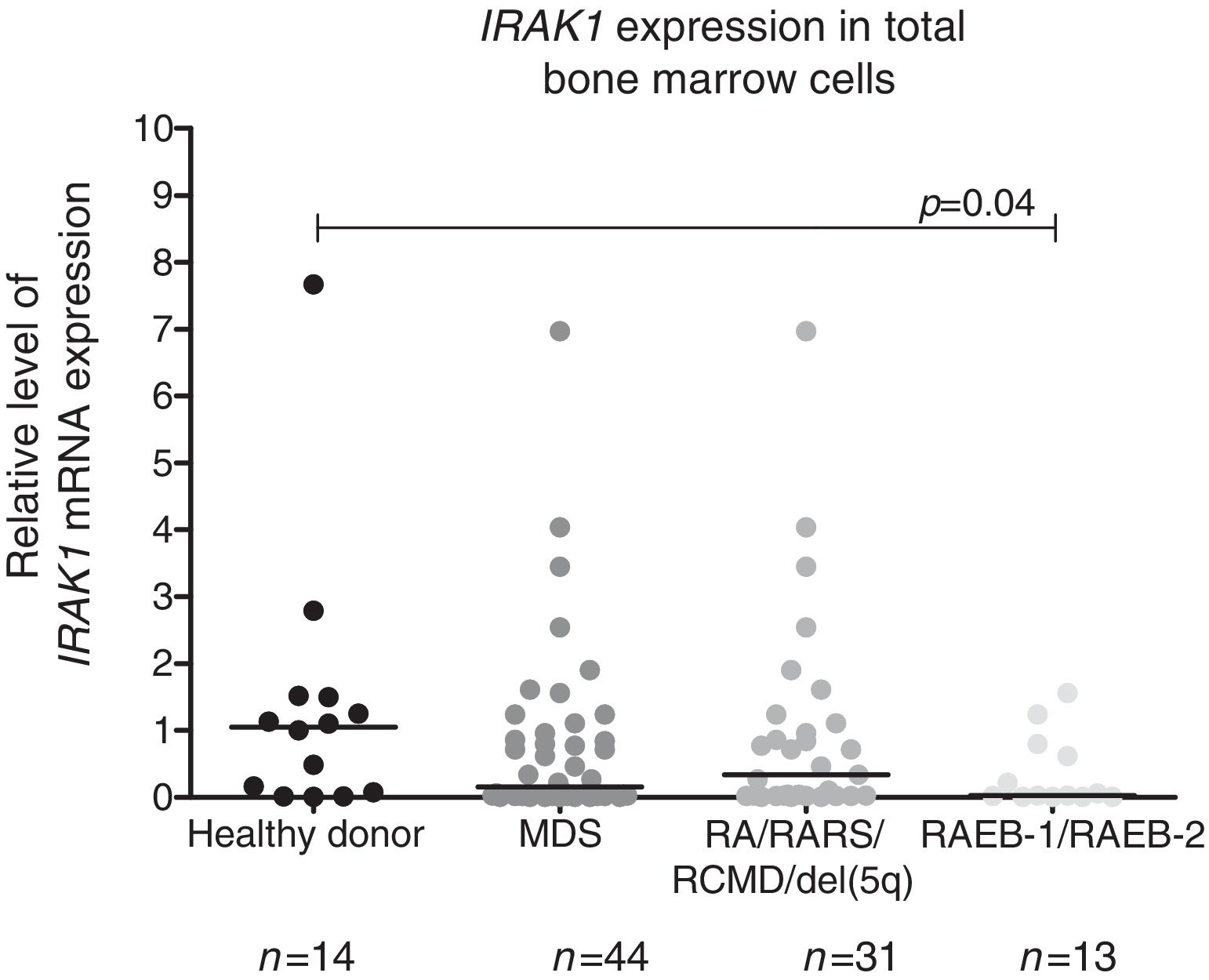
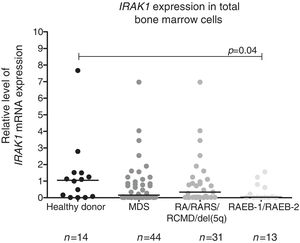
We observed a significantly lower IRAK1 mRNA expression in total bone marrow cells from refractory anemia with excess blasts (RAEB)-1/RAEB-2 MDS patients compared to healthy donors (p-value=0.04). There was no statistically significant difference in IRAK1 mRNA expression between refractory anemia (RA)/refractory anemia with ring sideroblasts (RARS)/refractory cytopenia with multilineage dysplasia (RCMD)/del(5q) MDS patients and all MDS patients vs. controls according to WHO 2008 classification (Figure 1), or between different IPSS-R risk groups: (a) low/very low/intermediate vs. high/very high; (b) low/very low vs. intermediate/high/very high; (c) low/very low vs. intermediate vs. high/very high (all p-value>0.05 – data not shown).
Reduced IRAK1 levels in RAEB-1/RAEB-2 myelodysplastic syndromes (MDS). IRAK1 mRNA expression by quantitative polymerase chain reaction analysis in total bone marrow cells from healthy donors and patients with a diagnosis of MDS (all patients), refractory anemia (RA)/refractory anemia with ring sideroblasts (RARS)/refractory cytopenia with multilineage dysplasia (RCMD)/del(5q) MDS and refractory anemia with excess blasts (RAEB)-1/RAEB-2 MDS according to WHO 2008 classification. The HPRT1 gene was used as the reference gene and a healthy donor was used as a calibrator sample. Horizontal lines indicate medians. IRAK1 expression is significantly reduced in RAEB-1/RAEB-2 MDS patients when compared to healthy donors, as indicated (p-value=0.04).
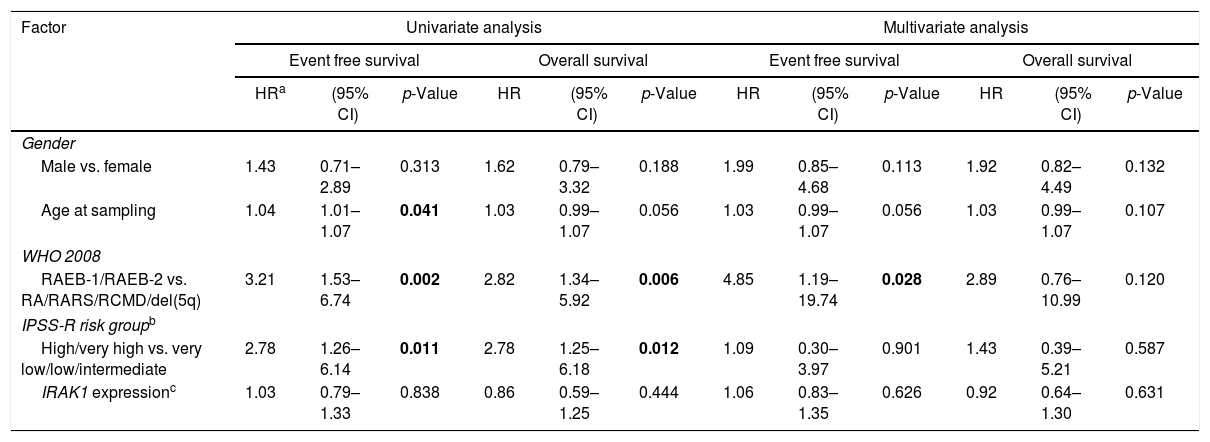
In order to assess the impact of IRAK1 transcript levels on EFS and OS in MDS, patients were categorized according to tertiles or median IRAK1 mRNA expression. There were no differences between the groups: lowest tertile vs. higher two tertiles; highest tertile vs. lower two tertiles; individuals above median vs. individuals below median (all p-value>0.05 – data not shown). The same was observed when IRAK1 mRNA expression was analyzed as a continuous variable (p-value>0.05). In our MDS cohort, RAEB-1/2 and high/very high IPSS-R risk predict poor EFS and OS by univariate analysis (p-value<0.02), increasing age predicts lower EFS by univariate analysis (p-value=0.041) and WHO 2008 classification was an independent predictor for EFS (p-value=0.028) (Table 2). IRAK1 mRNA expression did not associate with WHO 2008 classification, IPSS-R, hemoglobin values, platelet and neutrophil peripheral blood counts nor bone marrow blasts (Table 1).
Univariate and multivariate analyses of survival outcomes for myelodysplastic syndrome patients.
| Factor | Univariate analysis | Multivariate analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event free survival | Overall survival | Event free survival | Overall survival | |||||||||
| HRa | (95% CI) | p-Value | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | |
| Gender | ||||||||||||
| Male vs. female | 1.43 | 0.71–2.89 | 0.313 | 1.62 | 0.79–3.32 | 0.188 | 1.99 | 0.85–4.68 | 0.113 | 1.92 | 0.82–4.49 | 0.132 |
| Age at sampling | 1.04 | 1.01–1.07 | 0.041 | 1.03 | 0.99–1.07 | 0.056 | 1.03 | 0.99–1.07 | 0.056 | 1.03 | 0.99–1.07 | 0.107 |
| WHO 2008 | ||||||||||||
| RAEB-1/RAEB-2 vs. RA/RARS/RCMD/del(5q) | 3.21 | 1.53–6.74 | 0.002 | 2.82 | 1.34–5.92 | 0.006 | 4.85 | 1.19–19.74 | 0.028 | 2.89 | 0.76–10.99 | 0.120 |
| IPSS-R risk groupb | ||||||||||||
| High/very high vs. very low/low/intermediate | 2.78 | 1.26–6.14 | 0.011 | 2.78 | 1.25–6.18 | 0.012 | 1.09 | 0.30–3.97 | 0.901 | 1.43 | 0.39–5.21 | 0.587 |
| IRAK1 expressionc | 1.03 | 0.79–1.33 | 0.838 | 0.86 | 0.59–1.25 | 0.444 | 1.06 | 0.83–1.35 | 0.626 | 0.92 | 0.64–1.30 | 0.631 |
HR: hazard ratio; 95% CI: 95% confidence interval; WHO: World Health Organization; RA: refractory anemia; RARS: refractory anemia with ringed sideroblasts; RCMD: refractory cytopenia with multilineage dysplasia; del(5q): MDS with isolated del(5q); RAEB-1: refractory anemia with excess blast-1; RAEB-2: refractory anemia with excess blast-2; IPSS-R: revised international prognostic scoring system.
Statistically significant p-values are highlighted in bold.
An initial event selecting a clone of stem cells, together with abnormalities in immunosurveillance, could manifest with a higher propensity to cancer. Previous studies have described that defects in immune signaling might be involved in the pathophysiology of MDS.6,9–11 IRAK1 induces signaling through NF-κB and is negatively regulated by miR-146a, which is frequently expressed at low levels in MDS. Rhyasen et al.6 described the reduced cell proliferation of IRAK1-inhibited cells in vitro, and correlated high IRAK1 mRNA expression with reduced OS in MDS patients. In our cohort of MDS patients, however, IRAK1 mRNA expression did not predict survival outcomes nor differ when patients were categorized by WHO 2008 classification and IPSS-R. IRAK1 mRNA expression was not associated with blood counts and bone marrow blasts. Some differences in study design could explain the discrepancy between the results: firstly, our patient sample included a higher number of MDS patients (44 vs. 20); secondly, our study analyzed total bone marrow mRNA, while Rhyasen et al. analyzed CD34+ bone marrow cells.
In conclusion, although the deregulation of the IRAK1/TRAF6/NF-κB pathway may favor the development of MDS clones and the pharmacologic inhibition of IRAK1 might be helpful in inducing a cytostatic effect in MDS cells, IRAK1 mRNA expression in total bone marrow cells does not predict outcome in patients with MDS for the cohort studied.
FundingThis work was supported by the National Council of Technological and Scientific Development (CNPq), Instituto Nacional de Ciência e Tecnologia do Sangue (INCTS) and the São Paulo Research Foundation (FAPESP).
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank Andy Cumming for English review and Tereza Salles for her valuable technical assistance.