Diarrhea is frequently seen in autologous stem cell transplantation. Although toxicity related to conditioning is the most common cause, infectious pathogens can play a distinctive role particularly in certain regions and environments.
MethodsThe role of enteropathogens was investigated in 47 patients submitted to autologous stem cell transplantation at a Brazilian center between May 2011 and May 2013. All patients who presented with diarrhea consented to stool sample analysis to identify the etiological agents including coccidia, Strongyloides sp., Clostridium difficile and other pathogenic bacteria.
ResultsThirty-nine patients (83%) had diarrhea, among whom seven (17.5%) presented with coccidia, three (7.5%) with Candida sp., one (2.5%) with C. difficile, and one (2.5%) with Giardia lamblia. There was a tendency toward a higher incidence of diarrhea in older patients (p-value=0.09) and those who received conditioning with lomustine, etoposide, cytarabine, and melphalan (p-value=0.083). Furthermore, the number of days of neutropenia was higher in patients with diarrhea (p-value=0.06).
ConclusionsThe high frequency of diarrhea caused by coccidia shows the importance of investigating and correctly identifying etiological agents and highlights the possible varieties of intestinal infections in patients who undergo autologous stem cell transplantation.
Diarrhea is one of the most prevalent causes of morbidity in patients who undergo autologous stem cell transplantation (ASCT), resulting in a significant impairment to the patient's quality of life.1–3 The causes of diarrhea are mainly related to the toxicity of high-dose chemotherapy schemes, irritation of the epithelia and mucosa of the digestive tract and various infectious conditions.1
Clostridium difficile bacteria is a known cause of diarrhea of nosocomial origin4 in addition to being associated with the use of broad-spectrum antimicrobials.5 There is also a strong association between intestinal infections and opportunistic germs that are present and largely described in groups of immunocompromised patients submitted to chemotherapy as well as those with oncological diseases or acquired immunodeficiency syndrome (AIDS), such as strongyloidiasis, cryptosporidiosis, isosporiasis, and candidiasis.6
The empirical use of antimicrobials to treat these microorganisms is common practice in some ASCT centers.1 However, there are few studies regarding the etiology or the infections that may be involved in diarrheal events, especially in Brazil. The objective of this study is to identify the main etiologies of diarrhea in our environment.
MethodsOnce approval of the study was granted by the institutional research and ethics committee, all patients evaluated and submitted to ASCT at the bone marrow transplantation unit of the University Hospital of the Universidade Federal de Juiz de Fora (UFJF) between May 2011 and May 2013 were enrolled in this study consecutively.
All patients were evaluated by a parasitological stool examination and treated according to the pathogen found prior to ASCT since no patient presented with diarrhea at the time of the transplant procedure. Diarrhea was defined as the presence of two or more watery or loose fecal discharges within a 24-hour period. Diarrhea was classified as infectious in nature in cases in which enteric pathogens, intestinal parasites, or fungi were isolated or in the case of a positive test for A/B toxin for C. difficile. Afebrile patients who presented with diarrhea with no isolated infectious agents were considered as having chemotherapy-induced diarrhea and the others were defined as having diarrhea of an unspecified cause.
Parasitological stool examinations using the merthiolate-iodine-formalin and Baermann-Moraes techniques were conducted in three samples to assess the causes of infections in patients presenting with diarrhea. Fresh samples were collected for the direct assessment of fungi and coccidia as well as the assessment of the combined A and B C. difficile toxins using an enzyme-linked immunosorbent assay. Fresh stool cultures were performed for Shigella sp., Salmonella sp., and Escherichia coli. Other samples were collected from patients who stopped having diarrhea for 24hours but suffered further episodes at any time, and a new sample for C. difficile was collected from patients who presented with diarrhea and a new episode of fever.
All patients received prophylactic acyclovir from the beginning of the chemotherapy conditioning up to neutrophil engraftment without prophylaxis for bacteria and fungi. Cefepime was the empirical antimicrobial used initially for all patients in the event of fever during neutropenia.
Statistical analysis was conducted using Statistical Package for the Social Sciences (SPSS) software version 21, in which Pearson's chi-squared test or Fisher's exact test for categorical variables as well as the Mann–Whitney test for variables with non-normal distribution were used. p-values<0.05 were considered statistically significant.
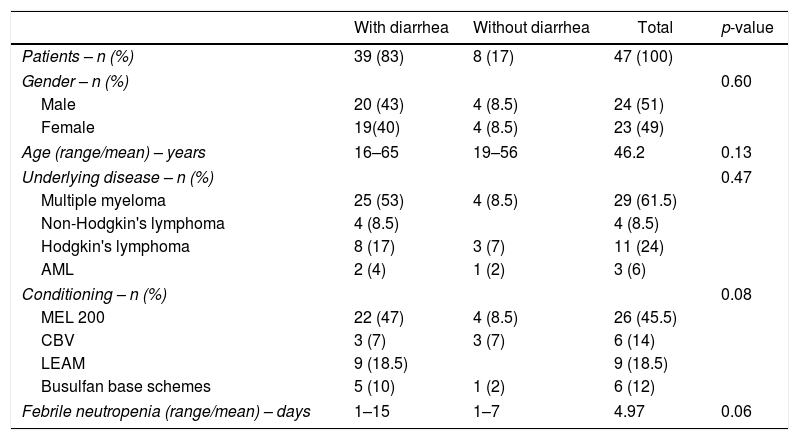
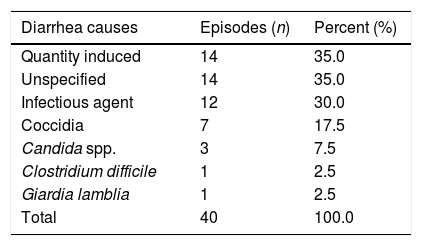
ResultsThis study enrolled 47 patients (Table 1). Among these patients, 39 had diarrhea, including one patient who had two distinct episodes giving a total of 40 events of diarrhea in the study period. Fourteen patients presented with diarrhea induced by chemotherapy, with no relationship between the conditioning chemotherapy protocol used and the presence of diarrhea, and 14 events were classified as having other causes. Among the 12 cases of infectious diarrhea, the most common agents were coccidia in seven cases, among which four cases were of Cryptosporidium sp. and in three cases it was not possible to determine the genus of the isolated coccidia. The second most frequent pathogen was Candida spp. in three cases, including one case where Cryptosporidium sp. was also isolated from the same sample. There was only one case of diarrhea caused by C. difficile and one case of Giardia lamblia (Table 2). All of the stool cultures were negative for enteropathogenic bacteria. Due to the small number of patients, it was decided not to correlate the causes of diarrhea with the underlying disease.
Patient, disease and transplantation characteristics.
| With diarrhea | Without diarrhea | Total | p-value | |
|---|---|---|---|---|
| Patients – n (%) | 39 (83) | 8 (17) | 47 (100) | |
| Gender – n (%) | 0.60 | |||
| Male | 20 (43) | 4 (8.5) | 24 (51) | |
| Female | 19(40) | 4 (8.5) | 23 (49) | |
| Age (range/mean) – years | 16–65 | 19–56 | 46.2 | 0.13 |
| Underlying disease – n (%) | 0.47 | |||
| Multiple myeloma | 25 (53) | 4 (8.5) | 29 (61.5) | |
| Non-Hodgkin's lymphoma | 4 (8.5) | 4 (8.5) | ||
| Hodgkin's lymphoma | 8 (17) | 3 (7) | 11 (24) | |
| AML | 2 (4) | 1 (2) | 3 (6) | |
| Conditioning – n (%) | 0.08 | |||
| MEL 200 | 22 (47) | 4 (8.5) | 26 (45.5) | |
| CBV | 3 (7) | 3 (7) | 6 (14) | |
| LEAM | 9 (18.5) | 9 (18.5) | ||
| Busulfan base schemes | 5 (10) | 1 (2) | 6 (12) | |
| Febrile neutropenia (range/mean) – days | 1–15 | 1–7 | 4.97 | 0.06 |
AML: acute myeloid leukemia; MEL 200: melphalan 200mg/m2; CBV: cyclophosphamide, carmustine, and etoposide; LEAM: lomustine, etoposide, cytarabine, and melphalan.
Distribution of the diarrhea causes in autologous stem cell transplantation at the institution.
| Diarrhea causes | Episodes (n) | Percent (%) |
|---|---|---|
| Quantity induced | 14 | 35.0 |
| Unspecified | 14 | 35.0 |
| Infectious agent | 12 | 30.0 |
| Coccidia | 7 | 17.5 |
| Candida spp. | 3 | 7.5 |
| Clostridium difficile | 1 | 2.5 |
| Giardia lamblia | 1 | 2.5 |
| Total | 40 | 100.0 |
There was no difference in the incidence of diarrhea in relation to gender, underlying disease, days of neutropenia, presence of fever, or use of antimicrobials. There was no statistical difference between a higher frequency of diarrhea and age of patients ≥50 years (p-value=0.09). All of the patients who received the LEAM (lomustine, etoposide, cytarabine, and melphalan)7 protocol developed diarrhea.
The patients who were specifically treated for the isolated diarrhea-causing agents had an average of 5.3 days of diarrhea, while those who were not treated specifically had an average of eight days of diarrhea (p-value=0.13).
There was no statistically significant correlation between febrile neutropenia and more days of neutropenia in patients with diarrhea (p-value=0.06); in eight of 12 infectious diarrhea events, patients had a neutrophil count <500μL when diarrhea began. There was no correlation between the use of antibiotics and the frequency of diarrhea considering that all patients took cefepime upon the development of fever and neutropenia, including the eight patients who did not present with diarrhea. Of the 39 patients with diarrhea, only 23% used cefepime before the diarrhea started. The use of other antimicrobials, such as vancomycin and carbapenem, had no correlation with the presence of diarrhea.
DiscussionDiarrhea is a very common event in patients submitted to ASCT, with frequencies in the literature of 47–91%.1,2,8,9 In our environment, 83% of the assessed patients presented with diarrhea, an important cause of morbidity that decreases the quality of life of these patients.3
Despite the varying frequencies in different reports in the literature,1,2,10C. difficile was uncommon as the cause of diarrhea in our environment, thus the empirical use of antimicrobial treatment with actions against this pathogen is not justified, even for patients using broad-spectrum antimicrobials such as fourth-generation cephalosporins for the treatment of febrile neutropenia.
Coccidia were present in approximately 18% of the patients with diarrhea, and this was the main cause of infectious diarrhea (58% of the isolated agents). These findings demonstrate a frequency that is above that reported in the literature for ASCT.1 This is probably due to its high prevalence in developing nations such as Brazil, in which the positivity rates in patients with AIDS are between 8.1 and 21.4%. There are some types of parasites that are endemic in developing countries and are associated with situations that lead to immunodeficiency.11,12
Despite showing no statistically significant difference, the diarrhea tended to be shorter in duration in those patients who were treated for an infectious agent compared to those who did not have an infectious cause, confirming the infectious etiology of the diarrhea in the patients who received antimicrobial treatment. One study showed that oral vancomycin is effective in almost 100% of critically ill patients infected with C. difficile.4
An investigation of infectious events conducted retrospectively in the same center,13 without performing a systematic study of the infectious agents in patients with diarrhea, identified only three cases of coccidia (two Cryptosporidium sp. and one Isospora sp.) in 72 patients with diarrhea. Therefore, a routine evaluation of pathogens in diarrheal stools is required, especially if the condition begins during the period of neutropenia as in 66.6% of the infectious diarrhea events in the current study; these patients had a neutrophil count <500μL.
Despite high-dose chemotherapy having been the main cause of diarrhea, in light of the fact that 30% of diarrhea episodes are related to infections and nearly 18% are caused by coccidia, the need to identify infectious agents to adequately treat diarrhea in patients submitted to ASCT is evident. Given these signs, clinicians must be attentive to the presence of coccidia in the environment.
Although this study involved a small number of heterogeneous patients, clinicians should be alert to diarrhea events in immunosuppressed populations.
Conflicts of interestThe authors declare no conflicts of interest.
We thank all those who contributed to data collection and analysis.